KEY POINTS
Conservative management of asymptomatic inguinal hernias is acceptable.
A proficient understanding of groin anatomy is essential to successful inguinal hernia treatment.
Elective repair of inguinal hernias can be undertaken using an laparoscopic or open approach.
The use of prosthetic mesh as a reinforcement significantly improves recurrence rates, whether the repair is open or laparoscopic.
Recurrence, pain, and quality of life are important outcome factors.
Laparoscopic inguinal hernia repair results in less pain and faster recovery, yet requires specialized training and equipment.
INTRODUCTION
Inguinal hernia repair is the most commonly performed operation in the United States, owing to a significant lifetime incidence and variety of successful treatment modalities. Approximately 800,000 cases were performed in 2003, not including recurrent or bilateral hernias.1 Advancements in perioperative anesthesia and operative technique have made this an outpatient ambulatory operation with low recurrence rates and morbidity. Given this success, quality of life and the avoidance of chronic pain have become the most important considerations in hernia repair.
Approximately 75% of abdominal wall hernias occur in the groin. The lifetime risk of inguinal hernia is 27% in men and 3% in women.2 Of inguinal hernia repairs, 90% are performed in men and 10% in women. The incidence of inguinal hernias in males has a bimodal distribution, with peaks before the first year of age and after age 40. Abramson demonstrated the age dependence of inguinal hernias in 1978. Those age 25 to 34 years had a lifetime prevalence rate of 15%, whereas those age 75 years and over had a rate of 47% (Table 37-1).3 Approximately 70% of femoral hernia repairs are performed in women; however, inguinal hernias are five times more common than femoral hernias. The most common subtype of groin hernia in men and women is the indirect inguinal hernia.4
Evidence of surgical repair of inguinal hernias can be traced back to ancient civilizations of Egypt and Greece.5 Early management of inguinal hernias often involved a conservative approach with operative management reserved only for complications. Surgery often involved routine excision of the testicle, and wounds were closed with cauterization or left to granulate on their own. Considering these procedures were performed before the advent of the aseptic technique, it is safe to assume that mortality was quite high. For those that survived the operation, recurrence of the hernia was common.
From the late 1700s to the early 1800s, physicians including Hesselbach, Cooper, Camper, Scarpa, Richter, and Gimbernat identified vital components of the inguinal region, and their contributions are reflected in the current nomenclature. Improved understanding of the anatomy and pathophysiology of inguinal hernias, coupled with the development of aseptic technique, led surgeons such as Marcy, Kocher, and Lucas-Championnière to perform sac dissection, high ligation, and closure of the internal ring. Outcomes improved, but recurrence rates remained high with prolonged follow-up.
Based on a comprehensive understanding of inguinal anatomy, Bassini (1844–1924) transformed inguinal hernia repair into a successful venture with minimal morbidity. The success of the Bassini repair over its predecessors ushered in an era of tissue-based repairs. Modifications of the Bassini repair were manifest in the McVay and Shouldice repairs. All three of these techniques, as well as modern variations such as the Desarda operation, are currently practiced.6
In the early 1980s, Lichtenstein popularized the tension-free repair, supplanting tissue-based repairs with the widespread acceptance of prosthetic materials for inguinal floor reconstruction. This technique was superior to previous tissue-based repair in that mesh could restore the strength of the transversalis fascia, thereby avoiding tension in the defect closure. Superior results were reproducible regardless of hernia size and type, and they were achievable among expert and nonexpert hernia surgeons alike.7
With the advent of minimally invasive surgery, inguinal hernia repair underwent its most recent transformation. Laparoscopic inguinal hernia repair offers an alternative approach, minimizes postoperative pain, and improves recovery. Since the initial description by Ger, the laparoscopic method has become significantly more sophisticated. Refinements in approach and technique have led to the development of the intraperitoneal onlay mesh, the transabdominal preperitoneal (TAPP) repair, and the totally extraperitoneal (TEP) repair. Furthermore, an array of prosthetic materials has been introduced to minimize recurrence and improve quality of life. Irrespective of the approach, successful surgical treatment of inguinal hernias depends on a sound grasp of inguinal anatomy.
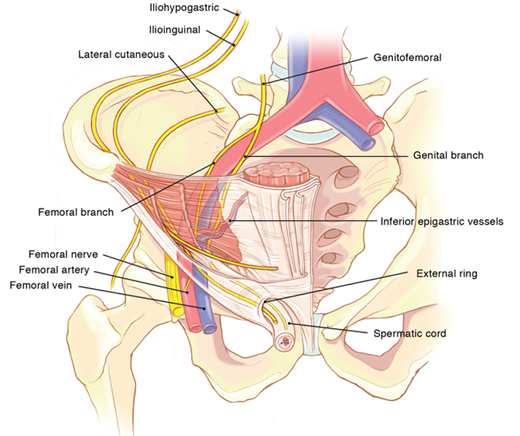
The inguinal canal is an approximately 4- to 6 cm-long cone-shaped region situated in the anterior portion of the pelvic basin (Fig. 37-1). The canal begins on the posterior abdominal wall, where the spermatic cord passes through the deep (internal) inguinal ring, a hiatus in the transversalis fascia. The canal concludes medially at the superficial (external) inguinal ring, the point at which the spermatic cord crosses a defect in the external oblique aponeurosis. The boundaries of the inguinal canal are comprised of the external oblique aponeurosis anteriorly, the internal oblique muscle laterally, the transversalis fascia and transversus abdominis muscle posteriorly, the internal oblique muscle superiorly, and the inguinal (Poupart’s) ligament inferiorly. The spermatic cord traverses the inguinal canal, and it contains three arteries, three veins, two nerves, the pampiniform venous plexus, and the vas deferens. It is enveloped in three layers of spermatic fascia.
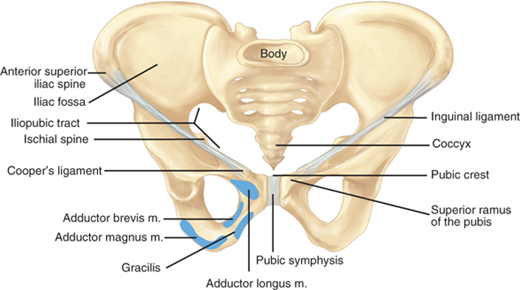
Additional important structures surrounding the inguinal canal include the iliopubic tract, the lacunar ligament, Cooper’s ligament, and the conjoined tendon (Fig. 37-2). The iliopubic tract is an aponeurotic band that begins at the anterior superior iliac spine and inserts into Cooper’s ligament from above. It forms on the deep inferior margin of the transversus abdominis and transversalis fascia. The shelving edge of the inguinal ligament is a structure that connects the iliopubic tract to the inguinal ligament. The iliopubic tract helps form the inferior margin of the internal inguinal ring as it courses medially, where it continues as the anteromedial border of the femoral canal. The lacunar ligament, or ligament of Gimbernat, is the triangular fanning of the inguinal ligament as it joins the pubic tubercle. Cooper’s (pectineal) ligament is the lateral portion of the lacunar ligament that is fused to the periosteum of the pubic tubercle. The conjoined tendon is commonly described as the fusion of the inferior fibers of the internal oblique and transversus abdominis aponeurosis at the point where they insert on the pubic tubercle.
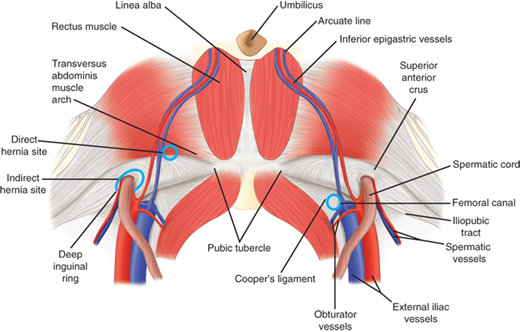
Inguinal hernias are generally classified as indirect, direct, and femoral based on the site of herniation relative to surrounding structures. Indirect hernias protrude lateral to the inferior epigastric vessels, through the deep inguinal ring. Direct hernias protrude medial to the inferior epigastric vessels, within Hesselbach’s triangle. The borders of the triangle are the inguinal ligament inferiorly, the lateral edge of rectus sheath medially, and the inferior epigastric vessels superolaterally. Femoral hernias protrude through the small and inflexible femoral ring. The borders of the femoral ring include the iliopubic tract and inguinal ligament anteriorly, Cooper’s ligament posteriorly, the lacunar ligament medially, and the femoral vein laterally. The Nyhus classification categorizes hernia defects by location, size, and type (Table 37-2).
| Type I | Indirect hernia; internal abdominal ring normal; typically in infants, children, small adults |
| Type II | Indirect hernia; internal ring enlarged without impingement on the floor of the inguinal canal; does not extend to the scrotum |
| Type IIIA | Direct hernia; size is not taken into account |
| Type IIIB | Indirect hernia that has enlarged enough to encroach upon the posterior inguinal wall; indirect sliding or scrotal hernias are usually placed in this category because they are commonly associated with extension to the direct space; also includes pantaloon hernias |
| Type IIIC | Femoral hernia |
| Type IV | Recurrent hernia; modifiers A–D are sometimes added, which correspond to indirect, direct, femoral, and mixed, respectively |
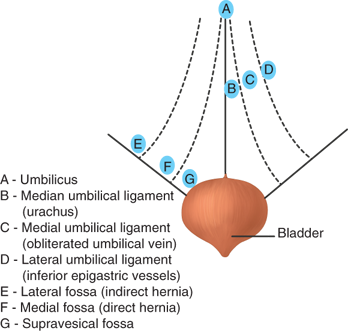
The laparoscopic approach to hernia repair provides a posterior perspective to the peritoneal and preperitoneal spaces (Fig. 37-3). Intraperitoneal points of reference are the five peritoneal folds, bladder, inferior epigastric vessels, and psoas muscle (Fig. 37-4). Two potential spaces exist within the preperitoneum. Between the peritoneum and the posterior lamina of the transversalis fascia is Bogros’s (preperitoneal) space. This area contains preperitoneal fat and areolar tissue. The most medial aspect of the preperitoneal space, that which lies superior to the bladder, is known as the space of Retzius. The posterior perspective also allows visualization of the myopectineal orifice of Fruchaud, a relatively weak portion of the abdominal wall that is divided by the inguinal ligament (Fig. 37-5).
Figure 37-4.
Posterior view of intraperitoneal folds and associated fossa: A. Umbilicus. B. Median umbilical ligament. C. Medial umbilical ligament (obliterated umbilical vein). D. Lateral umbilical ligament (inferior epigastric vessels). E. Lateral fossa (indirect hernia). F. Medial fossa (direct hernia). G. Supravesical fossa. (Modified with permission from Rowe JS Jr, Skandalakis JE, Gray SW. Multiple bilateral inguinal hernias. Am Surg. 1973;39:269.)
The vascular space is situated between the posterior and anterior laminae of the transversalis fascia, and it houses the inferior epigastric vessels. The inferior epigastric artery supplies the rectus abdominis. It is derived from the external iliac artery, and it anastomoses with the superior epigastric, a continuation of the internal thoracic artery. The epigastric veins course parallel to the arteries within the rectus sheath, posterior to the rectus muscles. Inspection of the internal inguinal ring will reveal the deep location of the inferior epigastric vessels.
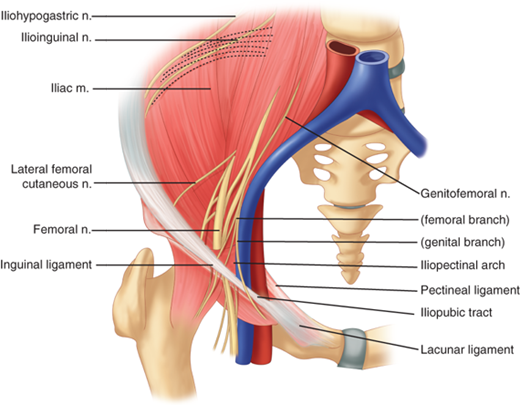
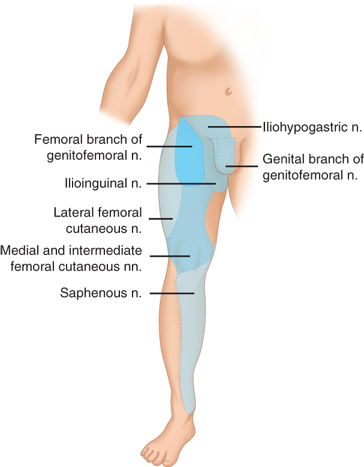
Nerves of interest in the inguinal region are the ilioinguinal, iliohypogastric, genitofemoral, and lateral femoral cutaneous nerves (Figs. 37-6 and 37-7). The ilioinguinal and iliohypogastric nerves arise together from the first lumbar nerve (L1). The ilioinguinal nerve emerges from the lateral border of the psoas major and passes obliquely across the quadratus lumborum. At a point just medial to the anterior superior iliac spine, it pierces the transversus and internal oblique muscles to enter the inguinal canal and exits through the superficial inguinal ring. It supplies somatic sensation to the skin of the upper and medial thigh. In males, it also innervates the base of the penis and upper scrotum. In females, it innervates the mons pubis and labium majus. The iliohypogastric nerve arises from T12–L1. After it pierces the deep abdominal wall, it courses between the internal oblique and transversus abdominis, supplying both. It then divides into lateral and anterior cutaneous branches. A common variant is for the iliohypogastric and ilioinguinal nerves to exit around the superficial inguinal ring as a single entity. The genitofemoral nerve arises from L1–L2, courses along the retroperitoneum, and emerges on the anterior aspect of the psoas. It then divides into genital and femoral branches. The genital branch enters the inguinal canal lateral to the inferior epigastric vessels, and it courses ventral to the iliac vessels and iliopubic tract. In males, it travels through the superficial inguinal ring and supplies the ipsilateral scrotum and cremaster muscle. In females, it supplies the ipsilateral mons pubis and labium majus. The femoral branch courses along the femoral sheath, supplying the skin of the upper anterior thigh. The lateral femoral cutaneous nerve arises from L2–L3, emerges lateral to the psoas muscle at the level of L4, and crosses the iliacus muscle obliquely toward the anterior superior iliac spine. It then passes inferior to the inguinal ligament where it divides to supply the lateral thigh (Fig. 37-8).
The preperitoneal anatomy seen in laparoscopic hernia repair led to characterization of important anatomic areas of interest, known as the triangle of doom, the triangle of pain, and the circle of death (Fig. 37-9).8 The triangle of doom is bordered medially by the vas deferens and laterally by the vessels of the spermatic cord. The contents of the space include the external iliac vessels, deep circumflex iliac vein, femoral nerve, and genital branch of the genitofemoral nerve. The triangle of pain is a region bordered by the iliopubic tract and gonadal vessels, and it encompasses the lateral femoral cutaneous, femoral branch of the genitofemoral, and femoral nerves. The circle of death is a vascular continuation formed by the common iliac, internal iliac, obturator, inferior epigastric, and external iliac vessels.
Figure 37-9.
Borders and contents of the (A) triangle of doom and (B) triangle of pain. a. = artery; Ant. = anterior; br. = branch; Lat. = lateral; n. = nerve; v. = vein. (Modified with permission from Colborn GL, Skandalakis JE. Laparoscopic cadaveric anatomy of the inguinal area. Probl Gen Surg. 1995;12:13.)
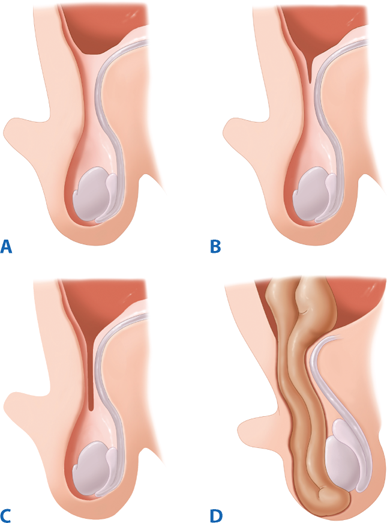
Inguinal hernias may be congenital or acquired. Most adult inguinal hernias are considered acquired defects in the abdominal wall although collagen studies have demonstrated a heritable predisposition. A number of studies have attempted to delineate the precise causes of inguinal hernia formation; however, the best-characterized risk factor is weakness in the abdominal wall musculature (Table 37-3). Congenital hernias, which make up the majority of pediatric hernias, can be considered an impedance of normal development, rather than an acquired weakness. During the normal course of development, the testes descend from the intra-abdominal space into the scrotum in the third trimester. Their descent is preceded by the gubernaculum and a diverticulum of peritoneum, which protrudes through the inguinal canal and becomes the processus vaginalis. Between 36 and 40 weeks of gestation, the processus vaginalis closes and eliminates the peritoneal opening at the internal inguinal ring.9 Failure of the peritoneum to close results in a patent processus vaginalis (PPV), hence the high incidence of indirect inguinal hernias in preterm babies. Children with congenital indirect inguinal hernias will present with a PPV; however, a patent processus does not necessarily indicate an inguinal hernia (Fig. 37-10). In a study of nearly 600 adults undergoing general laparoscopy, bilateral inspection revealed that 12% had PPV. None of these patients had clinically significant symptoms of a groin hernia.10 In a group of 300 patients undergoing unilateral laparoscopic inguinal hernia repair, 12% were found to have a contralateral PPV, which was associated with a fourfold 5-year incidence of inguinal hernia.11
| Coughing |
| Chronic obstructive pulmonary disease |
| Obesity |
| Straining |
| Constipation |
| Prostatism |
| Pregnancy |
| Birthweight <1500 g |
| Family history of a hernia |
| Valsalva’s maneuver |
| Ascites |
| Upright position |
| Congenital connective tissue disorders |
| Defective collagen synthesis |
| Previous right lower quadrant incision |
| Arterial aneurysms |
| Cigarette smoking |
| Heavy lifting |
| Physical exertion |
The presence of a PPV likely predisposes a patient to the development of an inguinal hernia. This likelihood depends on the presence of other risk factors such as inherent tissue weakness, family history, and strenuous activity. Overall, there are limited data regarding the etiology of inguinal hernia development. Several studies have documented strenuous physical activity as a risk factor for acquired inguinal hernia.12,13 Repeated physical exertion may increase intra-abdominal pressure; however, whether this process occurs in combination with a PPV or through age-related weakness of abdominal wall musculature is unknown. A case-controlled study of over 1400 male patients with inguinal hernia revealed that a positive family history was associated with an eightfold lifetime incidence of inguinal hernia. Chronic obstructive pulmonary disease also significantly increases the risk of direct inguinal hernias, as it is accompanied by repeated episodes of high intra-abdominal pressure.14 Several studies have suggested a protective effect of obesity. In a large, population-based prospective study of American individuals (First National Health and Nutrition Examination Survey), the risk of inguinal hernia development in obese men was only 50% that of normal weight males, whereas the risk in overweight males was 80% that of nonobese men. A possible explanation is the increased difficulty in detecting inguinal hernias in obese individuals.15
Epidemiologic studies have identified risk factors that may predispose to a hernia. Microscopic examination of skin of inguinal hernia patients demonstrated significantly decreased ratios of type I to type III collagen. Type III collagen does not contribute to wound tensile strength as significantly as type I collagen. Additional analyses revealed disaggregated collagen tracts with decreased collagen fiber density in hernia patients’ skin.16 Collagen disorders such as Ehlers-Danlos syndrome are also associated with an increased incidence of hernia formation (Table 37-4). Recent studies have found an association between concentrations of extracellular matrix elements and hernia formation.17 Although a significant amount of work remains to elucidate the biologic nature of hernias, current evidence suggests they have a multifactorial etiology with both environmental and hereditary influences.
| Osteogenesis imperfecta |
| Cutis laxa (congenital elastolysis) |
| Ehlers-Danlos syndrome |
| Hurler-Hunter syndrome |
| Marfan’s syndrome |
| Congenital hip dislocation in children |
| Polycystic kidney disease |
| α1-Antitrypsin deficiency |
| Williams syndrome |
| Androgen insensitivity syndrome |
| Robinow’s syndrome |
| Serpentine fibula syndrome |
| Alport’s syndrome |
| Tel Hashomer camptodactyly syndrome |
| Leriche’s syndrome |
| Testicular feminization syndrome |
| Rokitansky-Mayer-Küster syndrome |
| Goldenhar’s syndrome |
| Morris syndrome |
| Gerhardt’s syndrome |
| Menkes’ syndrome |
| Kawasaki disease |
| Pfannenstiel syndrome |
| Beckwith-Wiedemann syndrome |
| Rubinstein-Taybi syndrome |
| Alopecia-photophobia syndrome |
DIAGNOSIS
Inguinal hernias present along a spectrum of scenarios. These range from incidental discovery to surgical emergencies such as incarceration and strangulation of the hernia sac contents. Patients who present with a symptomatic groin hernia will frequently report groin pain. Extrainguinal symptoms such as a change in bowel habits or urinary symptoms are less common. Inguinal hernias may compress adjacent nerves, leading to generalized pressure, localized sharp pain, and referred pain. Pressure or heaviness in the groin is a common complaint, especially at the conclusion of the day or following prolonged activity. Sharp pain tends to indicate an impinged nerve and may not be related to the extent of physical activity performed by the patient. Neurogenic pain may be referred to the scrotum, testicle, or inner thigh. Questions should be directed to elicit and characterize extrainguinal symptoms. A change in bowel habits or urinary symptoms may indicate a sliding hernia consisting of intestinal contents or involvement of the bladder within the hernia sac.
Important considerations of the patient’s history include the duration and timing of symptoms. Hernias will often increase in size and content over a protracted time. Much less commonly, a patient will present with a history of acute inguinal herniation following a strenuous activity. It is more likely that an asymptomatic inguinal hernia became evident once the patient experienced symptoms after an acute event. Questions should also be directed to characterize whether the hernia is reducible. Patients will often reduce the hernia by pushing the contents back into the abdomen, thereby providing temporary relief. As the defect size increases and more intra-abdominal contents fill the hernia sac, the hernia may become harder to reduce.
Physical examination is essential to the diagnosis of inguinal hernia. Asymptomatic hernias are frequently diagnosed incidentally on physical examination or may be brought to the patient’s attention as an abnormal bulge. Ideally, the patient should be examined in a standing position to increase intra-abdominal pressure, with the groin and scrotum fully exposed. Inspection is performed first, with the goal of identifying an abnormal bulge along the groin or within the scrotum. If an obvious bulge is not detected, palpation is performed to confirm the presence of the hernia.
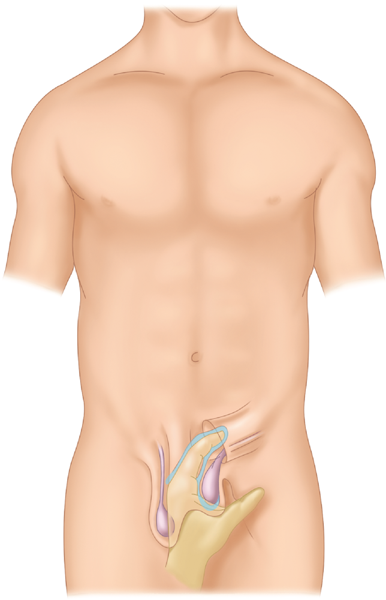
Palpation is performed by advancing the index finger through the scrotum toward the external inguinal ring (Fig. 37-11). This allows the inguinal canal to be explored. The patient is then asked to perform Valsalva’s maneuver to protrude the hernia contents. These maneuvers will reveal an abnormal bulge and allow the clinician to determine whether the hernia is reducible or not. Examination of the contralateral side affords the clinician the opportunity to compare the presence and extent of herniation between sides. This is especially useful in the case of a small hernia. In addition to inguinal hernia, a number of other diagnoses may be considered in the differential of a groin bulge (Table 37-5).
| Malignancy |
| Lymphoma |
| Retroperitoneal sarcoma |
| Metastasis |
| Testicular tumor |
| Primary testicular |
| Varicocele |
| Epididymitis |
| Testicular torsion |
| Hydrocele |
| Ectopic testicle |
| Undescended testicle |
| Femoral artery aneurysm or pseudoaneurysm |
| Lymph node |
| Sebaceous cyst |
| Hidradenitis |
| Cyst of the canal of Nuck (female) |
| Saphenous varix |
| Psoas abscess |
| Hematoma |
| Ascites |
Certain techniques of the physical examination have classically been used to differentiate between direct and indirect hernias. The inguinal occlusion test entails the examiner blocking the internal inguinal ring with a finger as the patient is instructed to cough. A controlled impulse suggests an indirect hernia, while persistent herniation suggests a direct hernia. Transmission of the cough impulse to the tip of the finger implies an indirect hernia, while an impulse palpated on the dorsum of the finger implies a direct hernia. When results of physical examination are compared against operative findings, there is a probability somewhat higher than chance (i.e., 50%) of correctly diagnosing the type of hernia.18,19 Accordingly, these tests should be used to detect hernias, but not to diagnose hernia types.
External groin anatomy is difficult to assess in obese patients, making the physical diagnosis of inguinal hernia challenging. A further challenge to the physical examination is the identification of a femoral hernia. Femoral hernias should be palpable below the inguinal ligament, lateral to the pubic tubercle. In obese patients, a femoral hernia may be missed or misdiagnosed as a hernia of the inguinal canal. In contrast, a prominent inguinal fat pad in a thin patient, otherwise known as a femoral pseudohernia, may prompt an erroneous diagnosis of femoral hernia.
In the case of an ambiguous diagnosis, radiologic investigations may be used as an adjunct to history and physical examination. Imaging in obvious cases is unnecessary and costly. The most common radiologic modalities include ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI). Each technique has certain advantages over physical examination alone; however, each modality is associated with potential limitations.
US is the least invasive technique and does not impart any radiation to the patient. Anatomic structures can be more easily identified by the presence of bony landmarks; however, because there are few bones in the inguinal canal, other structures such as the inferior epigastric vessels are used to define groin anatomy. Positive intra-abdominal pressure is used to elicit the herniation of abdominal contents. Movement of these contents through the canal is essential to making the diagnosis with US, and lack of this movement may lead to a false-negative result. A recent meta-analysis demonstrated that US detects inguinal hernia with a sensitivity of 86% and specificity of 77%.20 In thin patients, normal movement of the spermatic cord and posterior abdominal wall against the anterior abdominal wall may lead to false-positive diagnoses of hernia.21
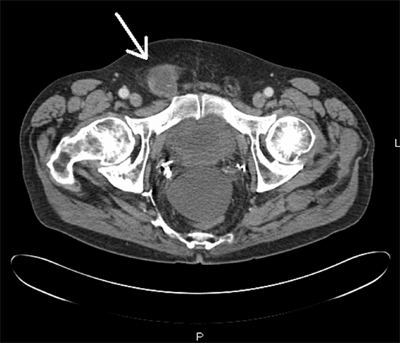
CT and MRI provide static images that are able to delineate groin anatomy, to detect groin hernias, and to exclude potentially confounding diagnoses (Fig. 37-12). A meta-analysis determined that standard CT detects inguinal hernia with a sensitivity of 80% and specificity of 65%. Although direct herniography has a higher sensitivity and specificity than CT, its invasiveness and limited availability restrict its routine use.20 As CT imaging increases in resolution, its sensitivity in detecting inguinal hernia is expected to expand; however, this has yet to be clinically confirmed by surgical correlation.22
When used to diagnose inguinal hernia, MRI is frequently reserved for cases where physical examination detects a groin bulge, but where US is inconclusive. In a 1999 study of 41 patients with clinical findings of inguinal hernia, laparoscopy revealed that MRI was an effective diagnostic test, with a sensitivity of 95% and specificity of 96%.23 MRI has become more sophisticated since 1999; however, its high cost and limited access remain obstacles to more routine use.
TREATMENT
Surgical repair is the definitive treatment of inguinal hernias; however, operation is not necessary in a subset of patients. When the patient’s medical condition confers an unacceptable level of operative risk, elective surgery should be deferred until the condition resolves, and operations reserved for life-threatening emergencies. Although the natural history of untreated inguinal hernias is poorly defined, the rates of incarceration and strangulation are low in the asymptomatic population. As a result, nonoperative management is an appropriate consideration in minimally symptomatic patients. Prospective studies and meta-analyses have demonstrated no difference in intent-to-treat outcomes, quality of life, or cost-effectiveness between nonoperative management and elective repair among healthy inguinal hernia patients.24,25 A 2012 systematic review found that 72% of asymptomatic inguinal hernia patients developed symptoms (mostly pain) and had surgical repair within 7.5 years of diagnosis.26 Nevertheless, the complication rates of immediate and delayed elective tension-free repair are equivalent.25,27 A nonoperative strategy is safe for minimally symptomatic inguinal hernia patients, and it does not increase the risk of developing hernia complications.
Nonoperative inguinal hernia treatment targets pain, pressure, and protrusion of abdominal contents in the symptomatic patient population. The recumbent position aids in hernia reduction via the effects of gravity and a relaxed abdominal wall. Trusses externally confine hernias to a reduced state and intermittently relieve symptoms in up to 65% of patients; however, they do not prevent complications, and they may be associated with an increased rate of incarceration.28 The risks of incarceration and strangulation appear to decrease over the first year, likely because gradual enlargement of the abdominal wall defect facilitates spontaneous reduction of hernia contents. The sheer volume of protruding tissue in an inguinal hernia does not necessarily signify severe morbidity.
Femoral and symptomatic inguinal hernias carry higher complication risks, and so surgical repair is performed earlier for these patients. Irrespective of symptoms, one study found the 3-month and 2-year cumulative incidences of strangulation were 2.8% and 4.5%, respectively, for inguinal hernias and 22% and 45%, respectively, for femoral hernias.29 Data from the Swedish Hernia Registry demonstrate that emergent operation is associated with a sevenfold increase in all-cause mortality over that of elective surgery among 107,838 groin hernia repairs.30 For this reason, it is recommended that femoral hernias and symptomatic inguinal hernias be electively repaired, when possible.
The administration of preoperative prophylactic antibiotics in elective inguinal hernia repair remains controversial. A systematic review of 7843 elective open hernia repairs from the Cochrane Database in 2012 revealed an overall decrease in infection rates (odds ratio [OR] 0.64, confidence interval [CI] 0.50–0.82) when prophylactic antibiotics are administered; however, the absolute risk reduction was not sufficient to justify universal recommendations for or against the practice. Overall wound infection rates are higher than those expected for clean operations, and there was a significant reduction in the rate of wound infection among patients undergoing repair with a prosthetic mesh.31,32 Although there is no universal guideline regarding the administration of prophylactic antibiotics for open elective hernia repair, it is our experience that meticulous perioperative protocol and surgical technique are more reliable countermeasures to prevent wound infection than antibiotics. Nevertheless, data trends and quality improvement measures have resulted in routine administration of prophylactic perioperative antibiotics in inguinal hernia repairs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree