Learning Objectives
Determine if an organism of interest is a bacterium, fungus, parasite, or virus and learn how it is further classified among related organisms.
Learn the organisms that produce the commonly encountered and better characterized infectious diseases.
Distinguish pathogenic organisms from those found in normal flora.
Learn the laboratory test results associated with the individual infectious diseases and how they are used in establishing the diagnosis.
Introduction
Humans live in a world of microbes. Many types of microbes are part of the normal human flora and rarely cause disease. Others have a greater potential for virulence and can cause disease depending on complex interactions between the host and the microbe. A small group of organisms are highly virulent and usually cause disease whenever they infect humans. This chapter on infectious diseases and clinical microbiology focuses on common pathogens and frequently encountered clinical syndromes. Infectious agents include a daunting array of viruses, bacteria, fungi, protozoans, and helminths. Table 5–1 provides information on the basic microbiology and clinical significance of common pathogens. The organisms are grouped based on shared properties because these are often relevant to the diagnostic process.
| Aerobic gram-positive cocci | Aerobic gram-negative bacilli | Mycoplasma and Ureaplasma | |
|---|---|---|---|
|
|
| |
| Virusesb | |||
| Family | Representative Species Pathogenic for Humans | Family | Representative Species Pathogenic for Humans |
|
|
|
|
|
|
| |
|
|
| |
Because of the large number of potential pathogens, it is not technically possible, practical, or cost-effective to attempt to rule out all of them in each patient who may have an infection. It is, therefore important for the clinician to know what organisms are most likely in a particular patient and whether routine diagnostic tests will detect them or whether specialized tests are needed. Identification of the causative agent is usually important for determining the most appropriate therapy. It can also have infection control or public health implications. The clinical findings are the first major clues in determining the site of infection and identifying a pathogenic organism. For example, a cough is usually indicative of a process in the respiratory tract while pain on urination is a clue to a urinary tract infection (UTI). Radiographic studies can further clarify the type of process and may point to specific categories of organisms.
Often, a single species of microbe can cause multiple syndromes. Conversely, a single syndrome may be caused by multiple organisms. This can lead to a potentially vast array of diagnostic possibilities. In order to determine the diagnosis in a timely and cost-efficient manner, it is essential to take into consideration the clinical setting. For example, the organisms responsible for a community-acquired pneumonia are usually different from those that cause nosocomial pneumonia. If the patient is immunosuppressed, this further enlarges the list of potential organisms. It also matters whether the immunosuppression is due to decreased cell-mediated immunity, for example, due to HIV infection or inhibitors of tumor necrosis factor (TNF), versus neutropenia secondary to chemotherapy because each has its own associated group of opportunistic infections. Other underlying conditions such as diabetes or sickle cell disease, or the presence of prosthetic devices, are associated with specific infections. A history of travel or exposure to arthropod vectors may raise the possibility of additional organisms.
The organization of this chapter reflects the common associations between selected organisms and the site of infection.
The organization of this chapter reflects the common associations between selected organisms and the site of infection. The discussion of a particular organism in a specific anatomic site in this chapter does not imply that infection by that organism is restricted to that location. Several infectious diseases, such as viral hepatitis (see Chapter 16) and Helicobacter pylori infections (see Chapter 15), are presented elsewhere in this book because these infections are intimately associated with a specific organ or tissue. The organisms and diseases selected for presentation in this chapter were chosen primarily by their incidence, with a preference for common infections. Many lower-incidence infections also have been included because they are often within a differential diagnosis, new information on their diagnosis and treatment is emerging, or they can be overlooked if appropriate tests are not requested. The chapter focuses on infections commonly encountered in the United States, although several travel-associated infections are discussed.
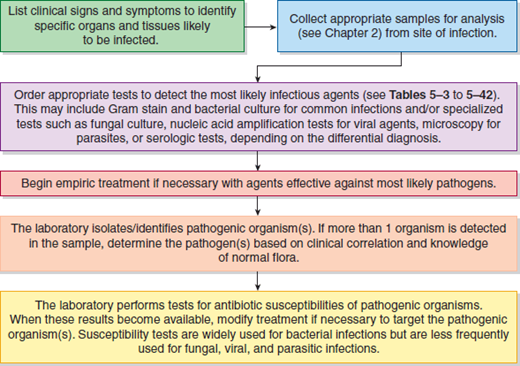
A general clinical approach to the patient with an infectious disease is diagrammed in Figure 5–1.
Laboratory Tests for Infectious Agents
The laboratory diagnosis of infectious disease utilizes 5 distinct types of tests: direct examination, culture, antigen detection, nucleic acid detection, and serology. Each of these tests has strengths and weaknesses. The types of test(s) that are used in a specific case depend in large part on the organisms that are in the differential diagnosis, as well as the type of specimens that are available. See Chapter 2 for illustrations of these laboratory methods.
Direct examination involves preparing a smear of the specimen and then using an appropriate staining technique to detect the relevant microorganisms. The Gram stain is rapid and detects most types of bacterial pathogens if they are present in sufficient numbers. It provides a preliminary characterization in terms of the Gram reaction, that is, positive or negative, as well as the morphology (cocci, coccobacilli, or bacilli) and arrangement of the cells (individual cells, pairs, chains, or clusters). It also provides information on the host response, for example, the presence or absence of neutrophils. This stain is routinely performed on most specimen types including respiratory specimens, sterile fluids, tissue biopsies, wounds, and abscesses. It is not routinely performed on stool because of the large numbers of normal flora, urine because similar information is available from the urinalysis, and blood because of the small number of organisms typically found in cases of bacteremia. The analytic sensitivity of the Gram stain is relatively low because the observation of an average of 1 organism per oil immersion field corresponds to a concentration of 105 organisms per milliliter in the specimen. The sensitivity can be increased by concentration of the specimen through centrifugation. Other direct stains include acid-fast stains for mycobacteria and calcofluor white for fungi. Wright stains of peripheral blood are used to detect Plasmodium and Babesia infections. Routine hematoxylin and eosin stains as well as Gram, acid-fast bacteria (AFB), and Gomori methenamine silver (GMS) stains can reveal the presence of microorganisms in paraffin-embedded tissue in the surgical pathology laboratory.
The laboratory diagnosis of infectious disease utilizes 5 distinct types of tests: direct examination, culture, antigen detection, nucleic acid detection, and serology.
Isolation of organisms in pure culture continues to be the mainstay of microbiologic diagnosis. In general, culture is very sensitive and specific and remains the “gold standard” for diagnosing many types of infection. Culture provides relatively rapid detection (within usually 1-3 days) of a wide array of organisms that are then available for definitive identification and antimicrobial susceptibility testing. One of the great advantages of culture assays is that microbiology laboratories routinely inoculate a combination of nonselective and selective media that will support the growth of many types of pathogenic bacteria. The person ordering the test does not need to specify which organism or organisms are suspected of causing the infection. A clinician who submits a blood-culture bottle does not have to order a Staphylococcus culture, a Streptococcusculture, a gram-negative rod culture, etc. Nonetheless, it is essential to remember that routine cultures only detect typical bacteria. If other classes of agents, such as mycobacteria, fungi, parasites, or viruses, are in the differential diagnosis, then the clinician must request the appropriate tests. For example, mycobacteria and fungal cultures utilize media designed to inhibit the growth of routine bacteria and they also require prolonged incubation.
The majority of bacterial pathogens are routinely identified by the presence of specific combinations of phenotypic traits including production of enzymes, such as catalase, coagulase, oxidase, and urease, ability to ferment or utilize different types of sugars, and additional metabolic reactions. Many of these individual tests have been combined into commercial identification kits. In contrast, slow-growing organisms, such as mycobacteria, and bacteria that are difficult to identify with traditional biochemical methods are now often identified by nucleic acid amplification and sequencing of conserved genes such as 16S ribosomal RNA or RNA polymerase. Yeasts are generally identified with biochemical tests similar to those used for bacteria. Finally, identification of molds is based on colonial and microscopic morphology.
More recently, commercial identification systems have been developed that use matrix-assisted laser desorption-ionization time-of-flight (MALDI-TOF) mass spectroscopy (MS) to generate protein spectra that can be compared with databases to identify bacteria and other microbes. The advantage of MALDI-TOF/MS is that it requires few reagents and can identify organisms in minutes as opposed to the 12- to 24-hour incubations required for most biochemical panels.
Once an organism has been identified in the clinical microbiology laboratory, the second major task, in most cases, is determining the organism’s antibiotic susceptibility profile. Susceptibility tests involve the measurement of the minimum inhibitory concentration (MIC), the lowest concentration of antimicrobial agent that inhibits the growth of the organism. The reference method is the microbroth dilution technique. The MICs are then interpreted as sensitive, intermediate, or resistant according to tables of interpretive breakpoints that are related to therapeutically achievable serum levels for each antibiotic. Other susceptibility methods such as disk diffusion (measuring the diameter of the zone of inhibition surrounding an antibiotic-containing disk) can be correlated with the results of the broth dilution technique.
Susceptibility testing has become increasingly complex due to the widespread dissemination of increasingly resistant pathogens that include methicillin-resistant Staphylococcus aureus (MRSA); vancomycin-resistant enterococci (VRE); and multidrug-resistant strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. These organisms harbor a variety of resistance mechanisms that include altered penicillin-binding proteins, extended-spectrum beta-lactamases, carbapenemases, inducible clindamycin resistance, and multidrug efflux pumps. While molecular methods can be used for rapid detection of specific resistance genes, such as the mecA gene in MRSA and the vanA and vanB genes in VRE, most susceptibility testing depends on phenotypic (MIC-based) methods that require overnight incubation.
Antigen detection tests do not require the growth of microorganisms. Therefore, they have the potential to provide rapid detection of infectious agents. Immunoassays that detect soluble antigens vary in speed, complexity, sensitivity, and specificity. Usually there is a trade-off between simplicity and sensitivity. Immunochromatographic assays require very few procedural steps and are the basis of many rapid point-of-care tests. These assays are less sensitive than traditional solid-phase enzyme immunoassays (EIAs) that are used for batch testing in the laboratory. Unlike culture-based assays, an immunoassay can only detect the organism that binds to the reagent antibodies; a separate assay must be performed for each organism. Immunoassays generally exhibit good but not perfect specificity. Furthermore, they cannot distinguish viable from nonviable organisms. Direct or indirect fluorescent immunoassays utilize microscopy to identify organisms that bind the reagent antibody. These assays often have high sensitivity and specificity compared with other types of immunoassays, but they require extensive training to insure proper interpretation.
The introduction of nucleic acid amplification techniques (NAATs) has revolutionized several areas of infectious disease testing including HIV viral load testing, diagnosis of Herpes simplex virus (HSV) encephalitis, and rapid detection of MRSA. They are particularly valuable for detecting difficult-to-grow or slow-growing organisms that may be present in small numbers. NAAT tests have the potential for very high sensitivity and specificity. However, as with antigen tests, they can only be used to diagnose infections caused by organisms that the assay detects. Current technology is not at the stage where it can eliminate the use of traditional culture methods in the routine bacteriology laboratory. Nonetheless, this is a rapidly changing field as illustrated by the widespread adoption of multiplex NAAT assays that can simultaneously detect a panel of common respiratory viruses.
Serologic tests detect host antibodies that are produced in response to infection with a particular infectious agent. The most important limitation of these assays is that antibody is usually not detectable early in the course of infection. Even if antibody is detected, it may represent a past infection. Serologic diagnosis usually requires demonstration of seroconversion, a 4-fold rise in IgG titer between sequential specimens, or a positive IgM assay. While the latter is usually thought of as a marker of acute infection, IgM can persist for 6 to 12 months. For the reasons outlined above, serologic assays are mainly used to diagnose infections that cannot be detected using more direct methods.
Sepsis and Bloodstream Infections
Normally the blood is sterile. Infection in any of the organs or tissues can result in entry of bacteria into the circulation. Replication of bacteria in the blood can contribute to the signs and symptoms of sepsis (eg, fever, tachycardia, leukocytosis, and hypotension) and may lead to dissemination of the organism to other tissues and organs; however, patients can be septic without having demonstrable bacteria in their blood. Bacteremia is often described as transient, intermittent, or continuous based on the number of positive specimens. Transient bacteremia occurs when small numbers of a commensal organism present on a mucosal surface gain access to the bloodstream. These infections are usually not clinically significant when they occur in an otherwise healthy host. Intermittent bacteremias are usually associated with a sequestered infection somewhere in the organs or tissues (eg, an abscess). Continuous bacteremias are associated with an intravascular focus of infection. Examples include endocarditis or an infected vascular catheter.
Blood cultures are routinely collected as part of the diagnostic evaluation of patients who present with signs and symptoms of sepsis or disseminated infection. To maximize sensitivity and specificity, it is recommended that 2 to 3 sets of blood cultures be collected per septic episode. Most hospitals use continuous blood culture systems that utilize colorimetric, fluorescent, or manometric methods to defect bacterial growth. Positive bottles are then subcultured to agar plates for further evaluation of the organisms.
To identify intermittent bacteremias, the timing of the blood collection is important to maximize the likelihood of finding organisms while they are in the blood (see the section on sample collection in microbiology in Chapter 2). Ideally, blood from patients with intermittent bacteremias is collected during the hour before a temperature spike, but this is not practical because the febrile episodes are often not predictable. It is common practice for blood to be collected at 30- to 60-minute intervals (if possible) when a febrile patient is suspected of having an intermittent bacteremia. As one might expect, one is much more likely to detect a continuous bacteremia in the first blood culture than to detect an intermittent bacteremia.
A major problem in the interpretation of the blood culture results is incidental contamination of the specimen with the normal bacterial flora from the skin.
A major problem in the interpretation of the blood culture results is incidental contamination of the specimen with the normal bacterial flora from the skin (Table 5–2). The clinical significance of a positive blood culture is dependent on both the number of positive specimens and the type of organism. The isolation of recognized pathogens such as S. aureus, Streptococcus pneumoniae, beta-hemolytic streptococci (Streptococcus pyogenesand Streptococcus agalactiae), enterococci, gram-negative rods (aerobic and anaerobic), or yeast from 1 or more blood cultures is almost always clinically significant (Table 5–3). In contrast, if only 1 of the multiple blood culture specimens is positive for an organism found on the skin (eg, coagulase-negative staphylococci or Corynebacterium spp.), the result is likely to reflect contamination during specimen collection rather than true septicemia. This frequently encountered problem is 1 reason why at least 2 blood samples should be collected for blood cultures. Although skin-derived bacteria are often thought of as nonpathogenic, it is important to remember that they can cause clinically significant infections, particularly in immunosuppressed patients and in patients with intravascular catheters or prosthetic devices. The isolation of the same skin flora organism in 2 separately collected specimens increases the probability that it represents a clinically significant bacteremia.
|
|
|
| Organism | Probability That the Organism Is a True Pathogena,b |
|---|---|
|
|
|
|
|
|
|
|
To avoid the problem of contamination of the blood culture bottles with skin organisms, meticulous preparation of the skin with a bactericidal agent is necessary. The number of blood cultures required for detection of a pathogenic organism is determined by the volume of blood collected per bottle, the timing of the blood collection, the type of organism producing the infection, and previous antibiotic exposure. Three or more blood culture collections may be required to document the presence of certain organisms.
To avoid the problem of contamination of the blood culture bottles with skin organisms, meticulous preparation of the skin with a bactericidal agent is necessary.
Infections Caused by Rickettsia, Ehrlichia, and Related Organisms
Rickettsia, Ehrlichia, and Anaplasma are obligate intracellular bacteria that cannot be detected in routine bacterial cultures. These organisms are transmitted to humans by ticks; therefore, the risk of acquiring these infections depends on the geographic distribution of the tick vectors and the time of year (see Table 5–4).
| Disease | Etiologic Agent | Mechanism of Transmission to Humans | Clinical Features | Laboratory Tests |
|---|---|---|---|---|
| Rocky Mountain spotted fever | Rickettsia rickettsii | Tick vector | Higher rate seasonally and in specific geographic areas; rash from a vasculitis, fever, and headache | Serology: increase in antibody titer after exposure; immunohistochemical tests give rapid result, but sensitivity is low at about 70% |
| Boutonneuse fever | Rickettsia conorii | Tick vector | Seasonal: distribution in Europe, Asia, and Africa; rash, fever, headache, and black spot at the site of tick attachment | Same as for Rocky Mountain spotted fever |
| Rickettsial pox | Rickettsia akari | Bite of mouse mite | Similar to but milder than Rocky Mountain spotted fever and Boutonneuse fever; uncommon in the United States | Serology: increase in antibody titer after exposure |
| Murine typhus | Rickettsia typhi | Flea feces inoculated into flea bite wound on human | Seasonal and geographic; rash, fever, and headache | Serology: increase in antibody titer after exposure |
| Epidemic typhus | Rickettsia prowazekii | Infected louse feces inoculated into human skin wounds | Associated with domestic crowding plus poor hygiene (as in refugee camps); clinically similar to Rocky Mountain spotted fever | Serology: increase in antibody titer after exposure |
| Scrub typhus | Orientia tsutsugamushi | Bite of a larval mite | Rash, fever, and headache | Serology: increase in antibody titer after exposure |
| Q fever | Coxiella burnetii | Inhalation of infected aerosols, ingestion of contaminated dairy products, or, rarely, by tick vector | Acutely, it is usually an asymptomatic or self-limited febrile pneumonia; can become chronic with damage to heart valves and bone | Serology: increase in antibody titer after exposure |
| Erhlichiosis | Ehrlichia chaffeensis | Tick vector | From asymptomatic to a severe Rocky Mountain spotted fever-like illness | Serology: increase in antibody titer after exposure; PCR |
| Anaplasmosis | Anaplasma phagocytophilium | Tick vector | Fever, headache, myalgias | Serology: increase in antibody titer after exposure; PCR |
Rickettsia rickettsii, the agent of Rocky Mountain spotted fever (RMSF), is mainly transmitted by the dog tick (Dermacentor variabilis) and infects endothelial cells. The resulting vascular injury elicits a widespread vasculitis, consisting of vasodilation with perivascular edema, and at times complicated by thrombosis and hemorrhage. Erythrocytes extravasating into the dermis form nonblanching petechial or purpuric lesions. The characteristic rash is often absent during the early stages of infection, and the infection can progress to a life-threatening encephalitis if not promptly treated.
Ehrlichia chafeensis is transmitted by the lone star tick (Amblyomma americanum) and infects monocytes. Patients present with nonspecific findings including fever, leukopenia, thrombocytopenia, and/or elevations of hepatic enzymes. Similar clinical manifestations are seen with Anaplasma (formerly Ehrlichia) phagocytophilum that is transmitted by deer ticks (Ixodes spp.) and infects granulocytes.
None of these agents can be cultured on artificial media. RMSF is usually diagnosed retrospectively with serologic tests; however, this should not delay treatment that should be initiated based on clinical findings and potential history of exposure. If the rash is present, organisms can be demonstrated by immunohistochemical staining of a skin biopsy in 70% of cases. Examination of peripheral blood smears in patients with Anaplasma can reveal the presence of organisms within inclusions in neutrophils, but many cases are negative. Ehrlichia infects monocytes but is rarely observed in peripheral blood smears. Polymerase chain reaction (PCR) of blood and/or serologic tests are the best methods for diagnosing Anaplasma and Ehrlichia infections.
Yeast belonging to the genus Candida are a major cause of hospital-acquired bloodstream infections. These organisms are frequently part of the oral and gastrointestinal flora. Treatment with broad-spectrum antibiotics that disrupt the normal bacterial flora, the presence of intravenous catheters, and neutropenia all predispose to the development of candidemia.
Cryptococcus neoformans and Histoplasma capsulatum are important causes of fungemia in patients with markedly depressed cell-mediated immunity (Cryptococcus is further discussed in the section “Chronic Meningitis” and Histoplasma is further discussed in the section “Infections of the Lung and Pleurae”). Although molds such as Aspergillus spp. can cause disseminated infections in immunosuppressed patients, they are rarely detected in the bloodstream.
Candidemia can usually be detected with routine blood cultures. Specialized techniques (eg, lysis–centrifugation cultures) are usually required to detect H. capsulatum and may enhance the detection of C. neoformans. Immunoassays that detect antigens produced by H. capsulatum and C. neoformans are also used to diagnose disseminated infections caused by these organisms.
Malaria is 1 of the largest causes of mortality and morbidity in the world. Individuals who travel to areas where malaria is endemic and develop fever within weeks of return should be suspected of suffering from malaria.
Several vector-borne parasites can infect the blood. These include protozoans such as Plasmodium spp. (malaria), Babesia spp., and Trypanosoma spp., and nematodes such as the agents of lymphatic filariasis. Plasmodium infections are an important cause of nonspecific febrile illnesses in returning travelers, and Babesia infections are endemic in the United States; these infections are discussed below. Other blood parasites are uncommon in the United States.
Malaria is one of the largest causes of mortality and morbidity in the world. Individuals who travel to areas where malaria is endemic and develop fever within weeks of return should be suspected of suffering from malaria.
There are 4 species of Plasmodium that cause most cases of human malaria. These parasites are transmitted by Anopheles mosquitoes that are widely distributed throughout Africa, Asia, and Latin America. The most dangerous of the 4 species is Plasmodium falciparum. This organism can achieve very high levels of parasitemia and adheres to capillary endothelium, and this can lead to severe organ damage. P. falciparum infection may be fatal within days. P. vivax and P. ovale are morphologically similar and generally cause less severe infection, but unlike P. falciparum, they can establish persistent infection and cause relapses several months after the initial infection. P. malariae is the least virulent species and can cause low-level infection that may cause few symptoms, but it can persist for years. P. knowlesi, which infects monkeys, can also cause human infections.
Currently, the diagnosis of malaria and the identification of each of the 4 species of Plasmodium responsible for malaria are based on the microscopic examination of stained erythrocytes in thick and thin blood films. These organisms have maturation cycles involving a variety of specific structures in the RBC, including ring trophozoites, growing trophozoites, mature schizonts, and gametocytes. The stippling of the RBCs with different dot patterns is also important in differentiating between the 4 species. Thus, the size and shape of the various malarial forms, their alteration of RBC morphology, and the stippling pattern in the RBCs provide the identification of the particular type of malaria. Quantitation of the level of parasitemia is also important. Marked parasitemia is a poor prognostic sign for P. falciparum infection. However, ill patients may have relatively low levels of parasitemia due to trapping of organisms in capillaries. PCR is starting to be used for the diagnosis of malaria and is particularly useful when low levels of parasitemia make it difficult to identify individual species. A rapid immunodiagnostic test for malaria may also be useful in settings where microscopy is not immediately available. Table 5–5 summarizes the relevant laboratory information for the diagnosis of malaria.
| Laboratory Test | Results/Comments |
|---|---|
| Identification of organisms within RBC in blood smears |
|
| Quantitation of parasitemia | Reported as percent of RBC parasitized or as number of parasites per 100 WBC; quantitation should be repeated after treatment to monitor effectiveness |
Babesia species are protozoa that, like Plasmodium species, infect erythrocytes. They are delivered to the infected host by the same tick (Ixodes) that transmits the agent of Lyme disease and human granulocytic anaplasmosis. Babesiosis mimics malaria in that it causes hemolysis, fever, anorexia, and hemoglobinuria. In the United States, B. microti is responsible for most cases of human babesiosis. In Europe, B. bovis and B. bigeminahave been implicated as agents of human disease. Babesiosis occurs mainly in the Northeast and upper Midwest in the United States. It affects patients of all ages, but most cases occur during the sixth and seventh decades of life. The infection from Babesia tends to be self-limited. In most cases, it lasts from weeks to months, following an incubation period of 1 to 6 weeks. Mild symptoms, including malaise, fever, and headache, characterize the disease in normal hosts, but asplenic patients often develop severe infections with high levels of parasitemia.
Babesia are protozoa that, like Plasmodium species, infect erythrocytes. They are delivered to the infected host by the same tick (Ixodes) that transmits the agent of Lyme disease.
The laboratory diagnosis rests upon the identification of the Babesia organisms inside erythrocytes in stained thick and thin peripheral blood smears. There are a number of morphologic features that differentiate Babesia from Plasmodium. Despite its relative shortcomings, serologic testing for B. microti can be performed as noted in Table 5–6. The level of parasitemia with Babesia does not always correlate with the severity of symptoms.
| Laboratory Test | Results/Comments |
|---|---|
| Identification of organism in RBC on blood film | Primary method of diagnosis; differentiating features in infected RBC suggesting Babesia rather than Plasmodium include 1) a tetrad of structures that resembles a “Maltese cross” and 2) the absence of pigment granules |
| Indirect immunofluorescent testing for antibodies to B. microti | A titer of >1:64 is considered indicative of exposure to the organisms, and a titer of >1:256 is diagnostic for an acute Babesia infection; at titers <1:256, the result does not clearly differentiate between patients who were exposed in the past and those who are actively infected |
| PCR amplification | Useful for confirming identification in low-level infections or detecting very low numbers of organisms; not routinely available |
Many viruses such as varicella zoster virus (VZV), measles, enteroviruses, and arboviruses (such as West Nile virus) exhibit a viremic phase. These viruses also exhibit organ-specific manifestations and are discussed in other sections of this chapter. Cytomegalovirus (CMV), Epstein–Barr virus (EBV), and parvovirus B19 are discussed below because they are common viruses that can have a direct effect on the blood.
Most cases of mononucleosis are caused by EBV, a member of the herpesvirus family that infects B lymphocytes and causes them to proliferate. This in turn stimulates the proliferation of cytotoxic T cells that control the active infection but do not eradicate the latent state. Infection with EBV is extremely common, and most individuals have asymptomatic infections. Patients with infectious mononucleosis typically present with fever, sore throat, and enlarged cervical lymph nodes.
In addition to mononucleosis, EBV is associated with 2 types of human tumor (Burkitt lymphoma and nasopharyngeal carcinoma) and is responsible for lymphoproliferative disorders in patients with severe immunosuppression following organ transplantation or AIDS. It also causes oral hairy leukoplakia in HIV-infected patients.
The diagnosis of EBV–associated infectious mononucleosis is usually confirmed by a positive serum heterophile antibody test that detects the presence of antibodies that agglutinate horse or cow erythrocytes.
Large atypical lymphocytes (cytotoxic T cells) are usually present in peripheral blood smears of patients with infectious mononucleosis caused by EBV, but they are also found in many other infections. The diagnosis of EBV-associated infectious mononucleosis is usually confirmed by a positive serum heterophile antibody test that detects the presence of antibodies that agglutinate horse or cow erythrocytes. The heterophile test is often negative in young children or in patients with atypical presentations; EBV-specific serologic tests are especially important in establishing the diagnosis in these situations. Quantitation of EBV DNA in peripheral blood is important in the diagnosis and management of EBV-associated lymphoproliferative disease in solid organ and bone marrow transplant recipients.
The use of laboratory tests in the diagnosis of infectious mononucleosis is shown in Table 5–7.
| Laboratory Tests | Comments |
|---|---|
| Heterophile antibody tests | Heterophile antibodies are IgM antibodies reactive with antigens on the cells from multiple species, and on this basis are termed heterophilic; they are detected in agglutination assays with horse or sheep RBCs or by their capacity to induce an agglutination response on antigen-coated latex particles; in infectious mononucleosis, the heterophile antibody test result is positive approximately 1 week after the symptoms first appear; the highest titers appear in the second to third week of the illness; relatively high titers persist for up to 8 weeks |
| Antibodies to EBV-specific antigens | Although heterophile-negative infectious mononucleosis is uncommon, it does occur, mostly in young children; in these cases, a characteristic clinical picture and the presence of IgM against the EBV viral capsid antigen (VCA-IgM) or a rising titer of VCA-IgG can confirm acute infection, whereas a negative VCA-IgM and positive VCA-IgG and positive EBNA antibodies (EBV nuclear antigen) indicate past infection |
| WBC differential | Patients with mononucleosis typically have a mild-to-moderate leukocytosis after the first week, with more than 60% of the WBCs as lymphocytes and 10%-20% of all lymphocytes being atypical; the maximum percentage of atypical lymphocytes appears between days 5 and 10 after the onset of symptoms, with a decrease to normal by approximately 3 weeks in most patients |
CMV causes several clinical syndromes. It infects leukocytes, where it remains latent in immunocompetent individuals but readily reactivates in immunosuppressed individuals. CMV is a leading cause of opportunistic infections in transplant recipients and AIDS patients. In transplant recipients, it often presents as a nonspecific febrile illness, but it can also cause more invasive infections including esophagitis, hepatitis, colitis, pneumonitis, and retinitis, particularly in severely immunocompromised patients. CMV is also the most common congenital viral infection. It affects approximately 40,000 infants born each year in the United States. Hematogenous spread appears to be responsible for transmission of the virus to the fetus. Most congenital infections occur when the mother has a primary CMV infection during the pregnancy. Neonates can also acquire the infection from maternal breast milk. Approximately 10% of infants congenitally infected with CMV are symptomatic at birth. Common sites of involvement are liver, spleen, lungs, and central nervous system (CNS). Because specific antiviral therapy is available for treatment of these infants, rapid detection of CMV infection is necessary. Although most congenitally infected infants are asymptomatic at birth, approximately 10% to 15% of these will develop later problems such as hearing loss and other neurologic problems. In children and young adults, primary CMV infection can cause a mononucleosis-like illness.
CMV is the most common congenital viral infection. It affects approximately 40,000 infants born each year in the United States.
The detection of CMV in blood and tissues generally correlates with active disease, whereas detection of CMV in urine is not necessarily diagnostic of active CMV disease, even in immunocompromised patients. Quantitative PCR assays of CMV DNA in blood correlate with the likelihood of severe infection. Congenital CMV infection is established when CMV is isolated from the urine of neonates less than 3 weeks of age. CMV isolation from respiratory secretions of bone marrow transplant recipients is likely to be clinically significant because interstitial pneumonia is a complication of bone marrow transplantation. In AIDS patients, shedding of CMV in respiratory secretions does not always correlate with active infection. CMV serology is used to determine whether donors and/or recipients are latently infected with CMV. This has important implications for preventing subsequent infections. Diagnostic testing for CMV is summarized in Table 5–8.
| Laboratory Test | Result/Comment |
|---|---|
| Conventional cell culture | Detection of CMV infection typically requires 7-28 days with conventional viral inoculation of cell cultures; CMV can be isolated from a variety of specimens including blood, bronchoalveolar lavage fluid, urine, and tissue |
| Shell vial assay | This is a modification of the conventional cell culture methodology for more rapid viral detection; viruses are detected earlier using this technique than conventional cell culture because the specimen is inoculated onto the monolayer of cultured cells by low-speed centrifugation; this enhances the infectivity of the cultured cells; this assay can often provide a positive result in 1-2 days |
| CMV antigen testing | A test is available for the identification of CMV antigen in polymorphonuclear leukocytes using a monoclonal antibody directed at a specific CMV protein; the assay is semiquantitative and permits monitoring response to therapy |
| Enzyme immunoassay (EIA) | This test is used to detect antibodies to CMV; seroconversion from negative to positive or a significant rise in anti-CMV IgG titer provides evidence of infection; assays for anti-CMV IgM are available, but detection of anti-CMV IgM does not always indicate primary infection because the IgM can persist for up to 18 months; most adults are seropositive and therefore serologic tests have limited utility for diagnosis |
| Polymerase chain reaction (PCR)-based detection of CMV | DNA- and RNA-based detection is available for the detection of CMV in peripheral blood leukocytes and tissues; quantitative assays are important for diagnosis of active infection in immunosuppressed patients |
Parvovirus B19 is a small single-stranded DNA virus that is transmitted by respiratory droplets. It is a common cause of infection in children in whom it causes a distinctive rash known as fifth disease. In adults, it often causes a significant arthropathy. An unusual feature of this virus is that it replicates in erythroid precursor cells and causes a temporary cessation of RBC production until the virus is cleared by the immune system. In normal hosts this has little, if any, consequence, but in patients who have a chronic hemolytic anemia such as sickle cell disease or hereditary spherocytosis, the parvovirus B19 infection causes a transient aplastic crisis in which there is a profound drop in the hematocrit. Parvovirus B19 can cause a chronic anemia in immunocompromised patients who are unable to clear the virus. Intrauterine infection of the fetus can also cause a severe anemia that leads to congestive failure and hydrops fetalis.
The clinical features of infectious endocarditis, a microbial infection of the valvular or nonvalvular endothelium of the heart, depend on the type of organism, location, and type of valve.
Acute parvovirus infection can be confirmed by demonstration of IgM antibodies or detection of viral DNA by PCR. During a transient aplastic crisis, the reticulocyte count decreases to <0.1% even as the hematocrit is declining.
Endocarditis: Infection of the Heart
The clinical features of infectious endocarditis, a microbial infection of the valvular or nonvalvular endothelium of the heart, depend on the type of organism, location, and type of valve. Acute infective endocarditis can present with temperatures ≥103°F, shaking chills, rapid worsening of valve function, and a variety of septic embolic complications. Subacute bacterial endocarditis is characterized by a low-grade or absent fever (as a result of infection by low-virulence organisms), and a variety of nonspecific signs and symptoms such as anorexia, weight loss, and malaise. Acute bacterial endocarditis typically occurs on native heart valves. It is most often caused by virulent organisms such as S. aureus, beta-hemolytic streptococci, and less commonly by S. lugdunensis, enterococci, and S. pneumoniae. Subacute bacterial endocarditis is usually caused by viridans group streptococci, enterococci, and fastidious gram-negative rods from the oral cavity. Several difficult-to-grow organisms are associated with “culture-negative” endocarditis. Prosthetic valve endocarditis is often caused by coagulase-negative staphylococci but can also be caused by S. aureus, other skin flora, enteric gram-negative rods, and fungi.
There are a number of risk factors for endocarditis, particularly in the acute form. These include diabetes, alcoholism, intravenous drug use, malignancy, infections in other sites, and immunosuppression. Anatomic defects also predispose patients to the development of infectious endocarditis. Such defects include mitral valve prolapse, congenital or rheumatic heart disease, and calcific aortic stenosis. The worst prognosis among patients with valvular disease is associated with those who have aortic valve involvement. The mitral valve, however, is the most frequently involved. Most individuals with endocarditis are between 45 and 60 years of age.
The clinical and laboratory features of acute and subacute bacterial endocarditis are different (Table 5–9). It should be noted, however, that patients can present with a syndrome of intermediate severity between acute and subacute endocarditis, usually as a result of infection by organisms of intermediate virulence, such as Enterococcus species, Haemophilus species, and the Streptococcus bovis group. Laboratory confirmation of infective endocarditis usually involves isolation of the same organism from multiple blood cultures. Organisms are more likely to be isolated from blood cultures in patients who have acute bacterial endocarditis because they have a high-grade persistent bacteremia. If 3 sets of blood cultures are obtained, the blood cultures are positive in more than 99% of patients who have not received antibiotics. At least 2 sets of blood cultures should be obtained by separate venipunctures at presentation. The erythrocyte sedimentation rate is a nonspecific test that is almost always elevated in cases of endocarditis, but it is useful for monitoring the response to therapy. It is not uncommon to obtain negative blood cultures in patients who meet the clinical and echocardiographic criteria for infectious endocarditis. Many of these “culture-negative” cases are due to prior antibiotic therapy. In the past, the “HACEK” group of fastidious oral gram-negative rods was linked to culture-negative endocarditis, but these organisms are readily detected by modern blood culture systems. More recently, interest has focused on Coxiella burnetii, Bartonella spp., Tropheryma whipplei, and Brucella spp. as potential causes of “culture-negative” endocarditis. These agents can be detected by a combination of serologic and molecular methods.
| Differentiating Clinical Features | Acute Infective Endocarditis | Subacute Infective Endocarditis |
|---|---|---|
| Temperature | >102°F | <102°F |
| Osler nodes | No | Yes |
| Janeway lesions | Yes | No |
| Roth spots (in eye exam) | No | Yes |
| Laboratory tests | ||
| Organisms most often detected in blood culture | Highly virulent pathogens such as Staphylococcus aureus, Streptococcus pneumoniae, Pseudomonas aeruginosa, often from a recognizable focus of infection | Organisms of lower virulence such as viridans streptococci, enterococci, and Streptococcus bovis; many other organisms can cause infective endocarditis, but are difficult to identify because they may require selective media or long periods for growth |
| Urinalysis and urine culture | The presence of hematuria and a pathogenic organism in a urine culture, in the appropriate clinical context, is consistent with acute infective endocarditic | Urinalysis reveals hematuria, pyuria, RBC casts, bacteriuria, and proteinuria |
| Complete blood count findings | ||
| Erythrocyte sedimentation rate | ≥50 mm/h | ≥50 mm/h |
| Anemia (normochromic, normocytic) | No | Yes |
| Markedly elevated WBC count | Yes | No |
| Transesophageal echocardiography | >90% sensitivity in detecting vegetations on cardiac valves; it is also capable of identifying valvular perforation, regurgitation, and abscess formation in many patients | >90% sensitivity in detecting vegetations on cardiac valves |
Laboratory confirmation of infective endocarditis usually involves isolation of the same organism from multiple blood cultures. Organisms are more likely to be isolated from blood cultures in patients who have acute bacterial endocarditis because they have a high-grade persistent bacteremia.
Infections of the Central Nervous System
Many organisms can produce an infection within the CNS. The major sites of infection are the meninges and brain parenchyma. Most organisms gain access to the CNS by hematogenous spread or by direct extension from an adjacent site. Bacterial infections can cause acute meningitis or may lead to formation of a brain abscess. Viral infections can present as meningitis or encephalitis, but often both sites are involved and the infection is more appropriately described as meningoencephalitis. Both fungi and mycobacteria can cause chronic meningitis while several parasites can cause intracerebral mass lesions. Each of these syndromes is often associated with specific organisms, information that can be used to guide the diagnostic workup. Rational test ordering based on clinical presentation is particularly important in patients with CNS infections since diagnostic specimens are difficult to obtain and are often present in limited quantities. Table 5–10 provides information on organisms that are frequently described causes of meningitis and/or encephalitis.
| Disease/Organism | Age of Highest Incidence | Higher Incidence in | Culture Available? | Smear Used in Diagnosis of CSF | Other Tests of Potential Use |
|---|---|---|---|---|---|
| Bacterial meningitis | |||||
| Group B Streptococcus (Streptococcus agalactiae) | <1 month | Neonates | Yes | Gram stain | Rapid antigen detection, mainly useful for partially treated infection |
| Streptococcus pneumoniae | All ages >3 months | Hypogammaglobulinemia | Yes | Gram stain | Rapid antigen detection, mainly useful for partially treated infection |
| Escherichia coli and other gram-negative bacteria (75% of E. coli cases are K1 strains) | <1 month and >60 years | Immunocompromised | Yes | Gram stain | Rapid antigen detection for E. coli K1, mainly useful for partially treated infection |
| Listeria monocytogenes | <1 month and >60 years | Immunocompromised | Yes | Gram stain | |
| Haemophilus influenzae type b | 1 month to 5 years | Immunocompromised; unvaccinated | Yes | Gram stain | Rapid antigen detection, mainly useful for partially treated infection |
| Neisseria meningiditis | 1 month | Patients with complement deficiencies | Yes | Gram stain | Rapid antigen detection, mainly useful for partially treated infection |
| Mycobacteria, especially M. tuberculosis | ≥1 month | Immunocompromised | Yes | Acid-fast stain (rarely positive) | Nucleic acid amplification |
| Treponema pallidum | Adults | Tertiary syphilis patients | No | None | Several tests for syphilis available (see the section “Syphilis”) |
| Pseudomonas aeruginosa | All ages | Neurosurgical postoperative patients | Yes | Gram stain | |
| Staphylococcus aureus | All ages | Neurosurgical postoperative patients | Yes | Gram stain | |
| Coagulase-negative staphylococci | All ages | Neurosurgical postoperative patients | Yes | Gram stain | |
| Other streptococci | All ages | Neurosurgical postoperative patients | Yes | Gram stain | |
| Fungal meningitis | |||||
| Cryptococcus neoformans | Adults | Immunocompromised | Yes | India ink | Rapid antigen detection is much more sensitive than India ink |
| Coccidioides immitis | Adults | Immunocompromised; those living in the southwestern United States, parts of Latin America | Yes | Calcofluor smear | Serologic testing of serum and CSF |
| Histoplasma capsulatum | Adults | Immunocompromised; those living in the Ohio and Mississippi River Valleys, and parts of Central America | Yes | Calcofluor smear | Serum, urine, or CSF antigen test; serum test for antibody to organism |
| Viral meningitis/encephalitis/meningoencephalitis | |||||
| Enteroviruses (includes echovirus and coxsackievirus) | All ages including infants | Late summer and early fall | Yes—viral culture using throat swab, and stool; CSF culture less sensitive than RT-PCR | None | RT-PCR of CSF specimen is the preferred diagnostic test |
| Herpes simplex virus-1 | All ages including infants | Can be primary or reactivation infections | Culture of CSF has low yield (can be performed on brain biopsy) | None | PCR of CSF specimen is the preferred diagnostic test; serologic testing; histochemical staining of brain biopsy |
| Herpes simplex virus-2 | Neonates | Infant of infected mother | Culture of CSF in neonates | None | PCR of CSF specimen is the preferred diagnostic test; serum test for antibody to virus |
| Cytomegalovirus | All ages | Immunocompromised | Shell vial culture of CSF or tissue | None | PCR of CSF; antigen detection in circulating WBCs; serum test for antibody to virus |
| Arboviruses | Peak age group varies for the different viruses in this group | Depending on the virus in the group, specific geographic regions and seasons for higher infection rates because transmission is by insects, usually mosquitoes or ticks | Rarely useful | None | Serum and/or CSF tested for antibody to specific viruses; RT-PCR available for some viruses (eg, West Nile) |
| Rabies virus | All ages | Individuals bitten or scratched by rabies-prone animal | Rarely useful | None | Immunofluorescence of brain biopsy specimen; testing of serum and CSF for antibodies to virus; RT-PCR on saliva |
| Measles virus | Childhood | Nonvaccinated individuals recently exposed to measles infection | Rarely useful | None | Testing of serum for antibodies to virus |
| Mumps virus | Childhood | Nonvaccinated individuals recently exposed to mumps infection | Yes—viral culture of throat swab, CSF, and urine | None | Testing of serum for antibodies to virus |
| HIV | Adults | Patients with AIDS or unexplained opportunistic infections | Rarely useful | None | Assays for diagnosis and monitoring of HIV |
| Varicella zoster virus | All ages | Immunocompromised; exposure to recent varicella zoster infection | Rarely useful | None | PCR of CSF specimen; testing of serum for antibodies to virus |
| Epstein–Barr virus | Children, adolescents, and young adults | Recent exposure to individual with infectious mononucleosis | Not available in clinical labs (research only) | None | PCR of CSF specimen; testing of serum for antibodies to virus |
Many organisms can produce an infection within the CNS. The major sites of infection are the meninges and brain parenchyma. Most organisms gain access to the CNS by hematogenous spread or by direct extension from an adjacent site.
Bacterial meningitis may present as a progressive illness over several days, or as a fulminant process that develops within hours. There is no single clinical sign that is pathognomonic of meningitis. Adolescents and adults typically present with combinations of fever, headache, nuchal rigidity, and other meningeal signs, and a decreased level of consciousness that can range from lethargy to coma; however, these findings are not present in all patients. Neonates and infants often present with nonspecific signs such as irritability, while nausea and vomiting are frequent complaints in children. Confusion, often without fever, is a common presenting sign in the elderly.
In most cases, the bacteria responsible for meningitis are acquired through the upper respiratory tract and then invade the blood. From the blood, they can then seed the meninges. There are a variety of factors that increase the risk for development of meningitis. These include splenectomy, sickle cell disease, cerebrospinal fluid leak, fistula or shunt, recent neurosurgical procedure, and infection contiguous to the CNS.
The organisms responsible for acute bacterial meningitis are highly dependent on the age of the patient and the clinical setting. S. agalactiae (group B Streptococcus [GBS]), E. coli, or Listeria monocytogenes are responsible for most cases of neonatal and infant meningitis. S. pneumoniae and N. meningitidis are responsible for most cases of community-acquired bacterial meningitis in children and adults (widespread vaccination for Haemophilus influenzae type b has nearly eliminated this previous childhood scourge). Elderly patients are at increased risk of infection with L. monocytogenes and aerobic gram-negative rods. In contrast, staphylococci and gram-negative rods are major causes of CNS shunt infections and postneurosurgery nosocomial infections. Knowledge of these patterns is important when deciding on empiric antibiotic therapy.
Examination and culture of CSF is essential. There is usually a markedly elevated WBC count with a preponderance of neutrophils, elevated protein, and decreased glucose (relative to the blood level). Gram stain of CSF reveals the causative organism in 70% to 90% of cases of pneumococcal and meningococcal meningitis. The percentage is generally lower for other bacteria. Bacterial culture is essential in all cases because it has the greatest sensitivity and specificity. Patients who have rapidly progressive or severe disease frequently receive a dose of antibiotics before a CSF specimen can be collected. Although this may cause a false-negative culture, it should have little or no effect on the cell count and differential, protein, glucose, and Gram stain. Immunoassays that detect S. pneumoniae and N. meningitidis capsular antigens in CSF are useful in these patients with partially treated meningitis, but they are not more sensitive than a Gram stain.
Viral meningitis (often described as aseptic meningitis due to the absence of bacteria) presents with fever, headache, and meningeal signs. There may be a mildly decreased level of consciousness. At least 75% of these infections are caused by members of the enterovirus family that includes the coxsackieviruses and echoviruses. Arboviruses (arthropod-borne viruses), HSV-2, HIV, and many other viruses can also cause this syndrome. Both enterovirus and arbovirus infections exhibit seasonal variation; most cases occur in the summer and early fall. Viral meningitis is usually a self-limited illness with a generally good prognosis. This is fortunate since there are currently no clinically useful antiviral drugs that are active against the enteroviruses and arboviruses. The clinical diagnosis of viral meningitis is often not clear-cut because there can be parenchymal involvement leading to varying degrees of encephalitis (see below). In addition, several other conditions can cause a similar clinical syndrome. These include partially treated bacterial meningitis, neoplastic diseases that have spread to the meninges, and immune-mediated diseases. It is important to identify these conditions because each of them is treatable and requires specific therapy.
Viral meningitis (often described as aseptic meningitis due to the absence of bacteria) presents with fever, headache, and meningeal signs. At least 75% of these infections are caused by members of the enterovirus family that includes the coxsackieviruses and echoviruses.
Acute bacterial meningitis must be differentiated from viral and fungal meningitis. There is a significant difference in the CSF findings between viral and bacterial meningitis (Table 5–11).
| Normal | Bacterial | Viral | Fungal or Tuberculous | |
|---|---|---|---|---|
| WBC (count/mL) | 0-5 | >100-5000 | 100-1000 | 50-500 |
| Neutrophils (% of total WBC) | 0-15 | >80 | <50b | <50 |
| Glucose (mg/dL) | 45-65 | <40 | 45-65 | 30-45 |
| CSF/blood glucose ratio | 0.6 | <0.4 | 0.6 | <0.4 |
| Protein (mg/dL) | 20-45 | >150 | 50-100 | 100-500 |
CSF analysis usually reveals an elevated WBC count with a preponderance of mononuclear cells, elevated protein, and a normal glucose. Identification of the causative agent is best achieved through the use of specific nucleic acid amplification tests or immunoassays; routine viral culture has a relatively poor yield in most cases. Many clinical or reference laboratories offer RT-PCR assays for enteroviruses, HSV, and West Nile virus. Immunoassays for detection of IgM and IgG are used to detect other viruses. Bacterial culture and cytopathology examination may be indicated if the diagnosis is unclear.
Patients suffering from chronic meningitis usually present with a variety of signs including low-grade fever, headache, lethargy, confusion, nausea, vomiting, and stiff neck that develop over a period of 1 to 4 weeks. Fungi and mycobacteria are responsible for many cases of chronic meningitis. The encapsulated yeast, C. neoformans, is 1 of the most common causes, particularly in patients with depressed cell-mediated immunity due to HIV infection or immunosuppressive therapy. C. neoformans is acquired by inhalation that usually causes an asymptomatic pulmonary infection. These organisms then spread to the CNS by the hematogenous route. C. gattii, previously classified as a subspecies of C. neoformans, often causes meningitis in patients who are not infected with HIV. Coccidioides immitis and C. posadasii, dimorphic fungi that are prevalent in the southwestern United States, are also acquired by inhalation and have a predilection for infecting the meninges and CNS. Immunosuppressed patients who harbor Mycobacterium tuberculosis are at increased risk of CNS tuberculosis (TB). Neoplastic and immune-mediated diseases can also cause chronic meningeal symptoms; it is important to distinguish between these conditions and infection.
The diagnosis of chronic meningitis requires evaluation of the CSF. Typically there are an increased number of mononuclear cells, mildly elevated protein, and normal glucose (except in TB). Immunoassays for C. neoformans capsular polysaccharide can be performed in less than an hour, have a very high sensitivity and specificity, and are superior to visual examination of India ink preparations (the antigen test does not distinguish between C. neoformans and C. gattii). Fungal culture should also be performed. If the patient is at increased risk of disseminated TB (ie, purified protein derivative [PPD]-positive or a history of pulmonary TB), then the CSF should be tested for mycobacteria. AFB smears are quite insensitive. PCR of CSF can provide early confirmation of M. tuberculosis infection in many cases. However, culture should still be performed to obtain an isolate for susceptibility testing.
Viral encephalitis is an infection of the brain parenchyma that can produce permanent neurologic damage or death in persons of all ages. A higher incidence of viral encephalitis is found in young children, the elderly, and persons with impaired immunity. For some viruses, there is a seasonal variation for infection. Most of the viruses that produce encephalitis enter the CNS via the hematogenous route. In its mildest form, viral encephalitis can present with fever and headache, and in its most severe form as an acute fulminating disorder with seizures and death. Prominent clinical findings include altered level of consciousness, altered mental status, headache, seizures, and other signs of neurologic dysfunction. The most important cause of sporadic encephalitis is HSV-1 that often causes permanent neurologic damage or death. Fortunately, there is effective antiviral therapy for HSV encephalitis that can prevent most of these complications if given early in the infection. Arboviruses transmitted by mosquitoes are responsible for periodic epidemics of encephalitis. A dramatic example of this phenomenon was the appearance of West Nile virus in the eastern United States in the summer of 1999 and its subsequent spread across the country during the next 4 years. Arboviral encephalitis is usually a self-limited infection. Most patients recover, but a significant number have persistent neurologic symptoms. Amebas such as Naegleria should also be considered in the differential diagnosis of encephalitis since they require specific therapy.
Viral encephalitis is an infection of the brain parenchyma that can produce permanent neurologic damage or death in persons of all ages. The most important cause of sporadic encephalitis is HSV-1 that often causes permanent neurologic damage or death.
The diagnosis of viral encephalitis includes evaluation of CSF. In patients with viral encephalitis, there is a predominantly lymphocytic pleocytosis, with a slight to moderate elevation of CSF protein, and no change in glucose content from normal. However, there are variations, depending on the virus that produces the encephalitis. Some of the agents can produce CSF findings that mimic those of bacterial meningitis (eg, pleocytosis with an increased number of neutrophils), particularly during the early stages of infection. The diagnosis of HSV encephalitis should be confirmed with a PCR assay of CSF that detects HSV DNA. This test is very sensitive and specific. Because of the severity of HSV infections and the availability of effective treatment, it should be performed on any patient with suspected encephalitis. Viral culture of CSF is insensitive and should not be routinely performed. Encephalitis caused by other viruses is often diagnosed by detection of viral nucleic acid or virus-specific IgM and IgG in serum or CSF.
Table 5–10 presents the laboratory evaluation for meningitis and encephalitis by disease and/or organism. Organisms not listed in the table also may cause CNS infections. Chapter 2 provides descriptions of many of the tests mentioned in the table.
A brain abscess is a focal lesion and therefore presents differently from meningitis and encephalitis. The most common clinical presentation is persistent, worsening headache. More than half of patients will have focal neurologic deficits, but only half have fever. Bacterial abscesses can result from hematogenous dissemination or extension from an adjacent site. The most common organisms are viridans group streptococci, Haemophilus spp., and anaerobic gram-negative rods. If the patient is immunosuppressed, then the abscess could be caused by Aspergillus and other fungi, Nocardia spp. and Mycobacterium spp., and Toxoplasma gondii. Neurocysticercosis, a mass lesion that results from infection with the pork tapeworm Taenia solium, often presents as new-onset seizures in an adult.
Unlike other CNS infections, examination of CSF is unlikely to yield useful information and even performing a lumbar puncture may be contraindicated. The initial diagnosis is usually based on CT and MRI findings. Identification of the causative agent is important for guiding therapy. This usually requires stereotactic biopsy of the abscess to obtain material for microscopic examination and culture. Serologic assays performed on serum may be helpful in the diagnosis of Toxoplasma infections and neurocysticercosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree