Hybrid Revascularization Strategies for Visceral/Renal Arteries
Benjamin W. Starnes
DEFINITION
The term “hybrid” in vascular surgery traditionally refers to the use of both traditional open surgical and endovascular techniques for remedy of the vascular condition (FIG 1).
Two hybrid approaches are described in this chapter.
Complete visceral debranching and endovascular tube graft repair
Partial visceral debranching and physician-modified fenestrated endovascular repair
DIFFERENTIAL DIAGNOSIS
Paravisceral aortic aneurysms may develop due to the following conditions:
Degenerative aneurysm
Aortic dissection
Mycotic aneurysm
Paraanastomotic juxtarenal aneurysm
Connective tissue disorders (Marfan’s syndrome)
Behçet syndrome
PATIENT HISTORY AND PHYSICAL FINDINGS
The majority of patients are asymptomatic and the diagnosis is made with imaging done for other reasons. Some patients will complain of mild to moderate abdominal and low back pain. Severe and unrelenting pain should raise the index of suspicion for a mycotic process which, if confirmed, would make hybrid approaches prohibitive.
IMAGING AND OTHER DIAGNOSTIC STUDIES
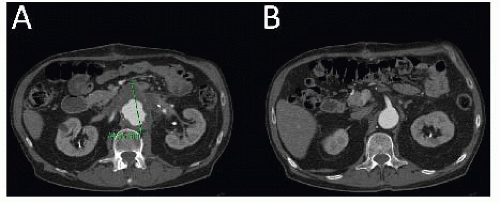
Contrast-enhanced, axial thin-slice computed tomography arteriography (CTA) is the current standard for imaging paravisceral aneurysms. Detailed information can be gathered regarding the precise origin of the celiac, superior mesenteric artery (SMA), and renal arteries (FIG 2).
Other important findings on CTA should be as follows:
Size and quality of access vessels for delivery of endovascular devices (>7 mm)
Location of left renal vein
Aberrant anatomy (e.g., replaced right hepatic artery)
Quality of gastroduodenal artery for possible celiac artery ligation or sacrifice
Renal cortical thickness
SURGICAL MANAGEMENT
Indications for repair include aortic aneurysms of more than 5.5 cm, symptoms, or evidence of rapid expansion (>0.5 cm per 6 months).
Preoperative Planning
As formal open repair would often include a bicavitary incision (chest and abdomen, as in a formal thoracoabdominal repair), the standard preoperative assessment should focus on the patient’s fitness to undergo major vascular surgery. This includes assessment of heart, lung, and kidney function and reserve.
Positioning
Proper and precise positioning should be as follows (FIG 3):
Patient supine on standard operating room table or imaging table
Hair properly clipped over entire abdomen and both groins
Both arms tucked (option to have right arm at 90 degrees if planning brachial access)
Foley under one leg and padded
TECHNIQUES
COMPLETE VISCERAL DEBRANCHING AND ENDOVASCULAR TUBE GRAFT REPAIR—STAGE 1
First Step—Exposure
Standard midline laparotomy and positioning of retractor system
Upon entry into the abdomen, the falciform ligament is divided between clamps and ligated. The triangular ligaments above the liver are divided to facilitate adequate exposure/retraction while minimizing risk of hepatic capsular injury, anticipating systemic anticoagulation later in the procedure.
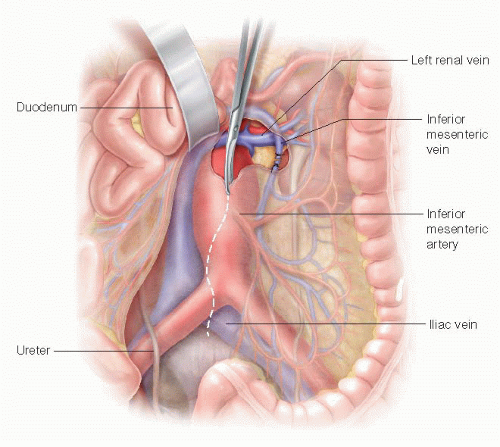
A nasogastric tube is positioned in the stomach to provide temporary decompression. The common hepatic artery is identified following division of the gastrohepatic ligament and traced back to origin of celiac artery. Once identified, the target artery is encircled with a silastic vessel loop. Space is created along the left side of the aorta with blunt/finger dissection, beginning at the level of the celiac artery, to create the retrograde bypass tunnel posterior to the pancreas (FIG 4).
The colon and omentum are lifted in a cephalad direction, the small bowel swept to the patient’s right and packed in moist towels. Self-retaining retractors (Omni or Bookwalter) should be positioned at this juncture to maintain exposure, with care taken to appropriately pad the retractor blades as necessary.
The third and fourth portions of the duodenum are mobilized to the right following division of the ligament of Treitz, exposing the anterior surface of the aorta. The inferior mesenteric vein is ligated and divided as well and the dissection continued along the proximal aorta until the left renal vein is clearly identified (FIG 5).
Widely mobilize the left renal vein sharply and encircle with a moist umbilical tape. The self-retaining renal vein retractor blade is used to retract the left renal vein cephalad as necessary to facilitate further exposure.
The origin of the renal arteries is identified by careful posterolateral dissection around the aorta, just cephalad of the overlying renal vein. Exposure on the right is complicated somewhat by the overlying inferior vena cava/left renal vein confluence. At least 2 cm of renal artery should be exposed bilaterally. Encircle the renal arteries with silastic vessel loops. On the left, finger dissect bluntly along the aorta in a cephalad fashion to complete the retropancreatic tunnel for the celiac limb of the bypass graft.
The SMA is identified next by palpation within the base of the small bowel mesentery, directly anterior to the pancreas. Doppler ultrasonography may assist identification when the pulse is faint. Once identified, a 3-cm segment of SMA is isolated as proximal as possible to the root of the mesentery. Beginning with the middle colic artery, multiple mesenteric arteries quickly branch from the SMA as it emerges from the pancreas, underscoring the need for proximal identification and isolation. The SMA is controlled with vessel loops.
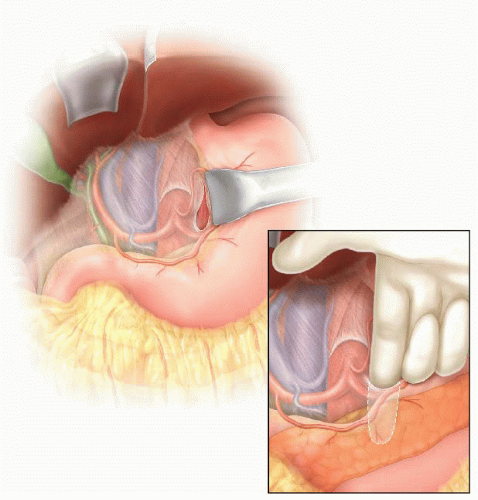
FIG 4 • Drawing of exposure of the celiac artery through the lesser sac. Note the blunt finger dissection along the left side of the aorta and behind the pancreas.
The next step is to prepare the donor artery for hybrid bypass. The specific artery—most commonly the common or external iliac arteries—should be selected from the preoperative imaging study. The retroperitoneum is opened directly over the selected donor artery, which is exposed while protecting the adjacent ureter. Alternatively, donor artery exposure may be achieved via medial-visceral rotation, developing the entire retroperitoneal plane on the left. The latter approach provides the added benefit of exclusion of the graft from the viscera and abdominal contents once the viscera are returned to their original position. This maneuver adds significantly more time to
the case, however, and contributes to increased blood loss. Graft coverage can also be obtained without developing the entire retroperitoneal plane, either via direct tunneling along the preferred course of the graft or creation of an omental tongue affixed directly to the graft.
Second Step—Anticoagulation
Systemic anticoagulation is achieved with a bolus injection of unfractionated heparin, 50 units/kg. Monitoring activated clotting time is a useful method of maintaining adequate anticoagulation during the procedure.
Third Step—Multivisceral Bypass
Trifurcated grafts exist for the purpose of facilitating multivessel hybrid revascularization, but the use of these are limited by the tendency of the middle limb to occlude when “squeezed” between the outside limbs during graft routing and abdominal closure. In most circumstances, a standard 12 × 7 bifurcated, collagen-impregnated knitted polyester graft provides excellent conduits for bilateral renal revascularization, with a separate 8-mm limb connected to the celiac and SMA. Examples of bypass graft configurations are shown in FIGS 6 and 7.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree