Hepatic- and Splenic-Based Renal Revascularization
Fred Weaver
Sung Wan Ham
Grace Huang
DEFINITION
The hepatic and splenic arteries represent suitable alternative inflow sources for renal artery revascularization. The most common indications for basing bypass procedures from these arteries include abdominal aortic occlusion or insufficiency or, alternatively, a scarred or hostile periaortic retroperitoneum. Hepatic- or splenic-based renal revascularization also minimizes increases in cardiac afterload induced by aortic crossclamping, which may be of benefit in patients with congestive heart failure. Alternative terms for hepatic- or splenic-based renal revascularization include hepatorenal bypass, splenorenal bypass, splanchnorenal bypass, or extraanatomic renal revascularization.
DIFFERENTIAL DIAGNOSIS
Renal revascularization is most commonly performed to alleviate “resistant” renovascular hypertension. Resistant hypertension is defined by a systolic blood pressure greater than 140 mmHg in patients taking at least three antihypertensive medications, representing 5% to 10% of all hypertensives. A subsegment of these patients has secondary hypertension due to renal artery pathology or endocrine tumors. Alternative causes of resistant hypertension include
Renal artery
Atherosclerosis
Aneurysm
Arteriovenous fistula
Fibromuscular dysplasia
Takayasu arteritis
Other vasculitides involving the renal artery (i.e., Behçet’s
syndrome, polyarteritis nodosa)
Trauma
Endocrine tumors associated with hypertension
Pheochromocytoma
Primary aldosteronism
Cushing’s syndrome
Primary adrenal hyperplasia
Hyperthyroidism
Acromegaly
PATIENT HISTORY AND PHYSICAL FINDINGS
Patient age: In younger patients, renovascular hypertension generally arises from nonatherosclerotic pathologies, such as Takayasu’s arteritis or fibromuscular dysplasia. In patients older than 50 years of age, atherosclerosis is most common etiology.
Associated risk factors are those typical for all occlusive arterial disease: tobacco use, diabetes, hyperlipidemia, and hypertension.
Length of the hypertensive diathesis: Was the hypertension easily controlled for a period of time, with a recent increase in the difficulty of control? Is the hypertensive diathesis severe and recent in onset? If either is true, the patient is more likely to have a secondary hypertension.
Recognition of the systemic burden of vascular disease present provides important perspective on indications and treatment options. Many vascular maladies involve multiple vascular beds. Is there evidence of disease involving the carotid artery, lower extremity arterial tree, and/or thoracic and abdominal aorta?
A history of postprandial pain, significant unintentional weight loss, and food avoidance is suggestive of mesenteric occlusive disease.
Prior pancreatitis may complicate attempts at splenic-based renal revascularization.
Prior Hodgkin’s disease or other neoplasms requiring mantle or midline abdominal radiation
For general operative risk considerations, recognition and documentation of the presence of coronary artery disease, previous coronary stents, or surgical coronary revascularization as well as valvular disease and congestive failure is fundamental to surgical planning.
Documentation of renal function as evidenced by increased serum creatinine, pedal edema, or recent requirement for renal replacement therapy
Recognition of prior aortic procedures, or intraabdominal nonvascular procedures such as a retroperitoneal lymphadenectomy for testicular cancer, which may complicate retroperitoneal dissection and aortic exposure Family history of syndromic aortic diseases such as Marfan’s, Ehlers-Danlos, and Loeys-Dietz
The specific antihypertensive regimen in place prior to surgery needs to be verified and documented.
To obtain the most accurate baseline measurement, the highest pressure obtained from either arm should be recorded and retained.
A complete vascular examination must be performed, with particular attention paid to pulse deficits and bruits. In particular, diminished femoral pulses or an abdominal bruit may indicate significant aortic or branch vessel occlusive disease, potentially complicating revascularization plans. The presence of concomitant carotid bruits may suggest carotid occlusive disease that should be assessed prior to renal revascularization. The presence of an aortic aneurysm should be excluded by abdominal palpation.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Laboratory assessment of renal function should include, at a minimum, serum creatinine, blood urea nitrogen (BUN), and electrolytes. Baseline glomerular filtration rate can be estimated from the serum creatinine level and body mass index using the Cockcroft-Gault equation.
The co-occurrence of endocrine syndromes, such as pheochromocytoma or functional adrenal tumors that potentially contribute to resistant hypertension should be evaluated with appropriate serologic studies.
Renal artery duplex ultrasonography is performed to document existing renal artery disease, renal mass, and intraparenchymal renal flow indices. Hemodynamically significant renal artery stenosis (>60%) are determined by duplex-derived assessment of peak systolic velocity measurements across lesions. Baseline characteristics (i.e., kidney size, velocity, spectral waveforms, resistive indices) serve as reference points for future surveillance imaging following revascularization.
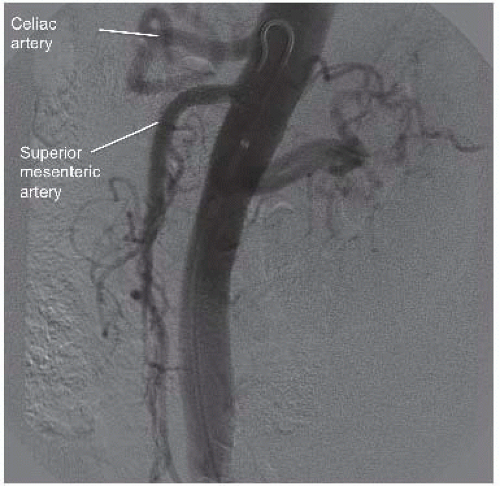
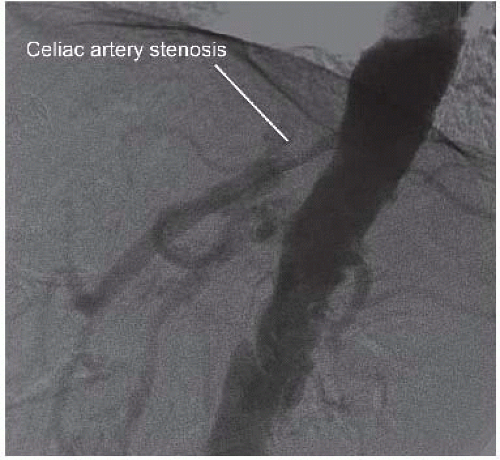
Selective visceral and renal arteriograms are obtained to define normal and variant vascular anatomy, including lateral imaging of both the celiac and superior mesenteric arteries (FIGS 1 and 2).
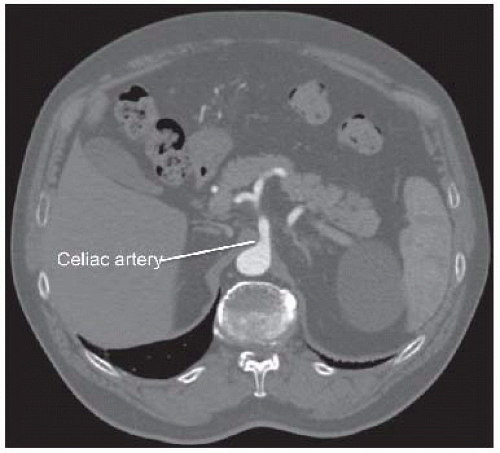
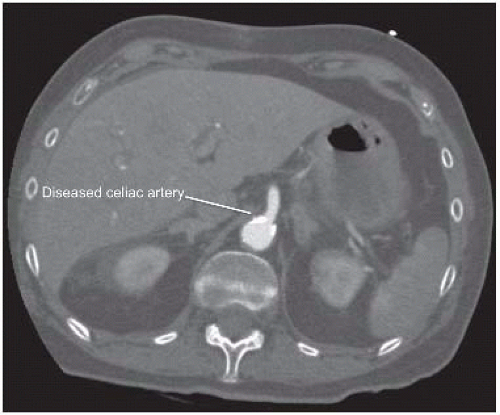
Computed tomography (CT) arteriography of the abdomen and pelvis, with arterial and venous pelvis, may provide additional useful information regarding the extent of aortic disease and other associated abdominal pathology (FIGS 3 and 4). Catheter-based arteriography alone may not identify significant arterial wall disease or the presence of aneurysmal lesions. However, the expense, contrast load, and radiation associated with complementary arteriographic imaging modalities may not be justified or appropriate in every patient, so anatomic information obtained from these examinations should be integrated into the operative plan on an iterative basis. Preoperative, imaging-based planning is combined with direct intraoperative assessment to create the most effective and durable revascularization possible for each patient.
Documentation of celiac, hepatic, splenic, and superior mesenteric artery patency is a mandatory prerequisite for these procedures. Significant stenosis of the celiac origin or hepatic or splenic artery occlusive disease will prevent successful renal revascularization from these arteries. Associated superior mesenteric artery disease also needs to be considered, particularly when the gastroduodenal artery provides significant collateral flow from the celiac plexus to the mesenteric bed. Renal artery anatomy, including branch vessel involvement and the presence of multiple renal arteries also needs to be documented.
Bilateral lower extremity vein mapping is also necessary to identify potential graft conduit. Standard vein mapping techniques, including imaging in a warm room with the patient in reverse Trendelenburg position, should be employed to ensure accuracy and reproducibility.
For selected patients, a more extensive preoperative evaluation for coronary artery or valvular disease should be considered. This may include both a transthoracic echocardiogram and cardiac stress evaluation. Selective pulmonary evaluation may be required in patients with chronic obstructive pulmonary disease (COPD)-associated respiratory
compromise. Additional vascular assessments should be performed as indicated, including carotid duplex ultrasonography to assess the significance of carotid bruits identified on physical examination.
SURGICAL MANAGEMENT
Preoperative Planning
The indications for hepatic and splenic artery-based renal revascularization are similar to those for aorta-renal revascularization and are discussed elsewhere.1,4
Although aorta-renal bypass is most direct and generally most expeditious, extraanatomic renal revascularization may be preferable in selected circumstances as previously noted.
Review of preoperative imaging is performed to determine variant vascular anatomy, if present. Anatomy of the existing renal artery disease is assessed.
The hepatic-right renal bypass requires a conduit, preferably autogenous vein.
The spleno-left renal bypass may be performed with or without graft conduit. The native splenic artery is sufficient length, usually to extend directly to the left renal artery, when fully mobilized. When necessary due to variant anatomy, or prior inflammation or scarring around the pancreas, venous conduit can also be employed.
Planning for availability of duplex ultrasonography in the operating room (OR) will facilitate intraoperative confirmation of adequate target revascularization and renal perfusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree