Healthcare-Associated Infections Related to the Use of Intravascular Devices Inserted for Short-Term Vascular Access
Angela L. Hewlett
Mark E. Rupp
BACKGROUND AND CLINICAL SIGNIFICANCE
Short-term vascular catheters are vitally important medical devices that are ubiquitously used in acute care settings. Over 150 million intravascular devices are purchased yearly in the United States, including 7 million central venous catheters (CVCs). Unfortunately, despite recent significant reductions in the incidence of IV catheter-related infections (1,2), they continue to result in substantial morbidity and excess economic cost. Although most studies that account for severity of illness have not found central line-associated bloodstream infection (CLA-BSI) to be independently associated with mortality (3,4,5), they do extend hospitalization by approximately 1 week (5) and result in excess attributable cost that ranges from $7,288 to $29,156 per episode (6). However, CLA-BSIs due to more virulent pathogens such as Staphylococcus aureus or Candida albicans are associated with greater morbidity and mortality than less virulent microbes such as Staphylococcus epidermidis (7,8).
There is a growing recognition that many, if not most, CLA-BSIs are preventable through the use of existing technology and clinical practice techniques, and there is increasing pressure to eliminate preventable CLA-BSI. Many states now require public reporting of hospital-specific CLA-BSI rates (9). The Centers for Medicaid and Medicare Services (CMS) no longer reimburses hospitals for excess costs associated with CLA-BSI (10) and the Department of Health and Human Services has recently targeted CLA-BSI for a 75% reduction within 5 years (11). These efforts have focused unprecedented scrutiny on CLA-BSI rates and fueled additional efforts to develop products and clinical techniques to prevent CLA-BSI.
EPIDEMIOLOGY
The National Healthcare Safety Network (NHSN) collects data on the incidence of healthcare-associated infections (HAIs) in the United States, including those related to the use of intravascular devices (12). The NHSN data are expressed as the risk of a CLA-BSI per 1,000 CVC days. The CLA-BSI rates from data collected from 2006 to 2008 are presented in Table 17-1. The risk of infection varied according to the type of ICU or inpatient ward setting, as well as the birth weight of the infant in neonatal ICUs. Burn units were found to have the highest rate of infection (5.5 per 1,000 catheter days) and pediatric medical ICUs had the lowest rate of infection (1.3 per 1,000 catheter days) (12). CLA-BSI rates are influenced by multiple factors including patient-related factors (severity and type of illness), catheter-related factors (catheter type and conditions under which the catheter was placed), and institutional factors including academic affiliation of the institution and bed size (13). It should be noted that the NHSN data may overestimate the true risk of CLA-BSI, because some bloodstream infections may be due to an unrecognized source
of infection that is unrelated to the CVC (13,14). In spite of these issues the risk-adjusted rates reported by NHSN are utilized by various types of facilities for benchmarking.
of infection that is unrelated to the CVC (13,14). In spite of these issues the risk-adjusted rates reported by NHSN are utilized by various types of facilities for benchmarking.
TABLE 17-1 National Healthcare Safety Network Central Line-Associated BSI Rates (2006-2008) | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
The European Centre for Disease Prevention and Control (ECDC) reported data from 695 hospitals in 12 European countries in 2007, which included 4,718 episodes of ICU-acquired bloodstream infections. These data indicate that ICU-acquired bloodstream infections occurred on average in 3% of patients staying more than 2 days in these ICUs, with 56% of these infections being catheter associated (15). The incidence of CLA-BSI in limited-resource countries has been investigated in a comprehensive review and is reported to range from 1.6 to 44.6 cases per 1,000 central line days in adult and pediatric ICUs (16). The International Infection Control Consortium (INICC) reported a CLA-BSI rate of 7.6 per 1,000 CVC days in INICC ICUs (17). These rates are higher than the rate reported by NHSN for the United States, which may be secondary to the lack of infection control resources in these developing countries. However, one study found the incidence of CLA-BSI in non-US and US hospitals to be similar in an international group of hospitals with similar infection control practices (18).
The type of intravascular device inserted markedly contributes to the risk of catheter-related bloodstream infections. Peripheral venous catheters are rarely associated with bloodstream infections. However, peripheral venous catheters are by far the most commonly used intravascular device, so the burden of bloodstream infections due to peripheral venous catheters may be more substantial than is commonly appreciated (19). Phlebitis is the most common complication associated with peripheral venous catheters and may represent inflammation rather than infection, but when phlebitis is present, the risk of subsequent bloodstream infection may be increased (20).
Studies show that midline catheters are associated with lower rates of infection than CVCs (21). Peripherally inserted central venous catheters (PICCs) have traditionally been utilized to administer medications in the outpatient setting, and have been shown to have a low infection rate (22). However, the use of PICCs is currently becoming more common in the inpatient setting. Infection rates in PICC lines inserted into high-risk hospitalized patients are associated with an infection rate similar to that of CVCs (23).
CVCs account for the vast majority of catheter-related bloodstream infections, causing an estimated 90% of these infections (20). Factors associated with increased risk of CLA-BSI include prolonged hospitalization, prolonged duration of catheterization, heavy microbial colonization at the insertion site or the catheter hub, internal jugular catheterization, neutropenia, prematurity, total parental nutrition, and substantial care (excessive manipulation) of the catheter (24). An increased risk of CLA-BSI has been observed with catheters with multiple lumens in observational studies, but a randomized trial did not observe a difference in infection risk between single lumen and triple lumen catheters (25).
The risk associated with site selection for CVC insertion has received particular attention in the medical literature. It has been suggested that the subclavian site is the preferred site of insertion in order to minimize the CVC infection risk, and femoral catheterization has been shown to be an independent risk factor for CLA-BSI (14). However, data on the optimal insertion site are conflicting, with some studies demonstrating a higher incidence of CLA-BSI when the femoral site is used, while others showing a higher incidence of CLA-BSI when the jugular site is used (26,27,28,29). One study showed that infection rates did not differ according to insertion site when experienced operators inserted the catheters, strict sterile technique was utilized, and trained ICU nursing staff performed catheter care (30). Another study involving patients requiring short-term dialysis vascular access demonstrated that the jugular site may be preferred over the femoral site in patients with a high body mass index (31). In contrast, several studies have demonstrated that the femoral site may be preferred in certain patients, especially those with tracheostomy (32, 33, 34). Pediatric studies have shown that placement of catheters at the femoral site in children have a low incidence of mechanical complications and that infection rates are equivalent to that of catheters placed at other sites (35, 36, 37). Careful consideration of the risks and benefits of placing a CVC at the recommended site should be weighed against the risks of mechanical complications, the experience of the person inserting the catheter, and patient-specific factors like the presence of open wounds, obesity, or anatomic deformity (13).
PATHOGENESIS
A comprehensive discussion of the pathogenesis of CLA-BSI is beyond the scope of this chapter and interested readers are directed to recent reviews (38,39). Briefly, the pathogenesis of intravascular catheter infections includes
complex interactions between the microbe, the catheter and what is infused through the catheter, and the host. Microbial factors include the presence of various polysaccharide and proteinaceous adhesions, and the ability to proliferate and elaborate biofilm. Catheter variables involve biomaterial composition, device design, and surface coatings. Host factors include such items as underlying illness, condition of the skin, local commensal flora, and site of catheter insertion.
complex interactions between the microbe, the catheter and what is infused through the catheter, and the host. Microbial factors include the presence of various polysaccharide and proteinaceous adhesions, and the ability to proliferate and elaborate biofilm. Catheter variables involve biomaterial composition, device design, and surface coatings. Host factors include such items as underlying illness, condition of the skin, local commensal flora, and site of catheter insertion.
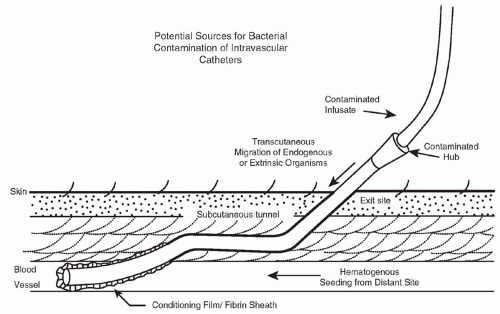
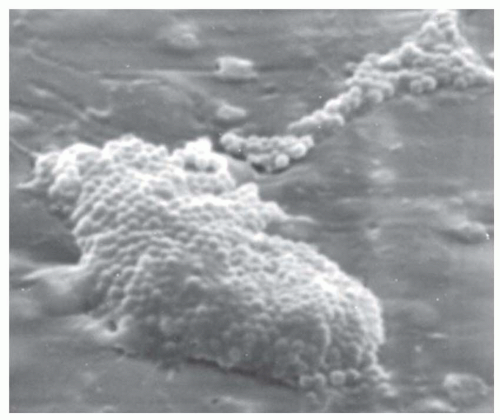
Figure 17-1 illustrates the routes by which microbes gain access to the catheter. For short-term, nontunneled, CVCs, it appears that most infections are caused by microorganisms from the patient’s skin that track via the external surface of the catheter (40). The longer a catheter remains in place, the more likely it is that a break in aseptic practices takes place and microbes gain access to the hub of the catheter and migrate via the luminal surface (41,42). Rarely do catheters become infected via hematogenous seeding or via infusion of intrinsically contaminated infusate (43). Once the microbes gain access to the catheter, they interact with the conditioning film that is comprised of host plasma proteins and blood cellular elements that quickly coat the catheter surface after insertion, and they adhere, proliferate, and elaborate biofilm (44). Figure 17-2 illustrates the appearance of a 1-day old, experimentally induced S. epidermidis biofilm-associated catheter infection. Physical and nutritional conditions differ from one portion of the biofilm to another and various populations of microbes are found, including rapidly growing cells at the macrocolony-blood interface as well as metabolically quiescent, antibiotic-resistant, persister cells in deeper portions of the biofilm (45). Because of associated cost and morbidity and because successful treatment of biofilm-associated catheter infections often requires removal of the catheter, it behooves us to apply stringent measures to prevent these infections in the first place.
MICROBIOLOGY
The 2008 NHSN Annual Update report contains data on pathogens associated with HAIs and the associated antimicrobial resistance patterns reported between January 2006 and October 2007 (46). Overall 463 hospitals reported one or more HAIs, resulting in a total of 28,502 HAIs including 10,064 (35.3%) cases of CLA-BSI.
According to the NHSN data, gram-positive cocci remain the most common cause of CLA-BSI. Coagulase-negative staphylococci are the most frequent causative microorganism in both adult and pediatric patients, causing 34.1% of the cases of CLA-BSI in the hospital setting (46, 47, 48). Other pathogens that cause CLA-BSI include Enterococcus sp. (16%), Candida sp. (11.8%), and S. aureus (9.9%). Gram-negative bacilli like Klebsiella sp., Escherichia coli, Enterobacter sp., Pseudomonas sp., and Acinetobacter sp. account for 17.7% of CLA-BSI according to NHSN data.
Antimicrobial resistant microorganisms are commonly implicated in CLA-BSI infections. Data from NHSN indicate that 56.8% of S. aureus isolates are resistant to oxacillin (MRSA), and 36.4% of Enterococci are vancomycin resistant (VRE). Resistance amongst gram-negative microorganisms is also emerging, with 27.1% of Klebsiella pneumoniae isolates demonstrating resistance to third generation cephalosporins, and 23% of Pseudomonas aeruginosa isolates demonstrating resistance to carbapenems such as imipenem and meropenem.
CLINICAL MANIFESTATIONS
Signs and symptoms related to intravascular device infections vary depending on whether the infection is localized or systemic. Local catheter infections include those at the exit site, tunnel infections, and phlebitis. Exit site infections commonly manifest with evidence of localized inflammation including erythema, warmth, tenderness, and purulent discharge. An infected CVC tunnel may demonstrate similar inflammatory signs extending along the tunneled portion of a tunneled catheter. Phlebitis is inflammation of the veins associated with a catheter. It typically involves peripheral catheters, and often presents with signs of local inflammation. Phlebitis may represent a localized infection or a chemical irritation of the vein due to the catheter material or drugs administered. Localized infections may lead to a systemic catheter infection, but are not a reliable predictor of such infections (49).
Systemic catheter infections typically present with signs of systemic infection, including fever, chills, and hemodynamic instability. Other signs may include altered mental status, catheter dysfunction, and complications of bloodstream infections such as endocarditis.
DIAGNOSIS
Initial diagnosis of catheter-related bloodstream infection involves examining the catheter site for local signs of infection and obtaining blood cultures. Since the symptoms and signs of CLA-BSI are nonspecific, microbiologic evidence is necessary to establish that the catheter is the source of the infection. Diagnostic methods to identify CLA-BSI can be divided into methods that require removal of the catheter and methods that do not necessitate removal of the catheter (50).
One diagnostic technique that does not require removal of the catheter involves obtaining paired blood cultures (one from the catheter and one through a peripheral vein) prior to initiation of antimicrobial therapy (50,51). When possible, these cultures should be drawn by a phlebotomy team (52). A definitive diagnosis of CLA-BSI can be made if the blood culture drawn through the catheter yields a colony count that is at least threefold greater than that of the culture obtained from the peripheral vein (51,53,54). If a peripheral blood culture is not possible, two quantitative blood cultures obtained through two catheter lumens in which the colony count for the blood sample drawn through one lumen is at least threefold greater than the colony count for the blood sample obtained from the second lumen is suggestive of CLA-BSI (51). One study demonstrated that obtaining blood samples from all catheter lumens may help to establish a diagnosis of CLA-BSI (55). However, this has not been clearly documented in the literature, and the possible advantages must be weighed against the additional time required, expense, and possibility of contributing to healthcare-associated anemia. If there is purulent material present at the catheter exit site, this should also be sent for Gram stain and culture (56).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree