General Concepts and Epidemiology
Before the patient safety movement began, the prevention of hospital-acquired infections was seen as the job of the hospital epidemiologist and other infection control staff, who tried (often unsuccessfully) to engage clinicians in prevention efforts. Branding healthcare-associated infections (HAIs) as a patient safety problem (which by extension rendered failure to engage in appropriate infection control practices a form of medical error) has elevated the importance of these infections and propelled prevention into the mainstream.
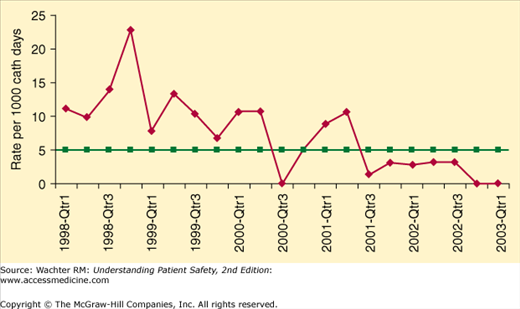
Gratifyingly, evidence is accumulating that healthcare organizations can markedly decrease the frequency of HAIs. Some hospitals, having religiously implemented a variety of prevention strategies, are reporting intervals of months, even years, between previously commonplace infections such as ventilator-associated pneumonias (VAP), methicillin-resistant Staphylococcus aureus (MRSA), and central line–associated bloodstream infections (CLABSI) (Figure 10-1).
Figure 10-1
Marked decrease in catheter-related bloodstream infections after implementation of safety practices at Johns Hopkins Hospital. (Reproduced with permission from Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med 2004;32:2014–2020.)
If we consider HAIs resulting from failure to adhere to evidence-based practices preventable adverse events (a position I endorse), then HAIs may well be the most common source of serious and preventable harm in healthcare. The Centers for Disease Control and Prevention (CDC) estimates that 1 in 10–20 hospitalized patients will develop an HAI, that HAIs are responsible for approximately 100,000 deaths per year in U.S. hospitals, and that these infections are responsible for $30 to 40 billion in attributable costs.1,2 Beginning in 2008, Medicare began withholding payments to hospitals for the care of certain HAIs (CLABSI, healthcare-associated urinary tract infections, and Clostridium difficile infections) it considered largely preventable (Chapter 20 and Appendix VIII).3
For many HAIs (and other complications of healthcare, see Chapter 11), a variety of processes or structural changes appear to be correlated with improvement. In the past, infection control experts and regulators have underscored the need to increase adherence to individual prevention elements—for example, if there were five strategies thought to be effective in preventing a certain type of infection, a hospital might get “credit” for achieving 100% adherence on one of the five elements, 80% on another, and 50% on the other three. The Institute for Healthcare Improvement (IHI) has promoted a “bundle” approach, emphasizing that the chances of preventing complications seem to improve with complete adherence to a “bundle” of preventive strategies.4 Under this model, institutions receive credit for their quality and safety efforts only for above-threshold adherence (i.e., >80%) on all of the preventive strategies, not just some. This approach rests on the theory that not only does better adherence to the individual elements increase the chances of prevention but also achieving high bundle adherence rates usually requires reengineering the entire clinical process of care. Changes born of this kind of reengineering may be more durable than those that result from short-term projects or cheerleading. This theory, though conceptually attractive and useful as a motivational tool, has not been empirically validated.
By this point in the book, you won’t be surprised to learn that successfully eradicating HAIs is not as simple as creating and promoting the use of an evidence-based bundle of practices. In an insightful study, Saint et al. surveyed 86 people from a variety of disciplines (physicians, hospital executives, nurses, infection control preventionists, and others) to determine the barriers to successful implementation of evidence-based HAI prevention strategies.5 They identified two kinds of personnel—active resistors and “organizational constipators”—who impeded these efforts. To illustrate the resistor category, the authors quote one hospital chief of staff, who gave the following advice to those charged with getting surgeons to follow HAI guidelines:
Surgeons are very tribal so if you, what you need to do, is you have something that you think is a best practice at your hospital . . you need to get . . either the chair of surgery or some reasonable surgeon . . If you come in and you’re an internist . . into a group of surgeons . . the first thing we’re going to do is we’re going to say, “Look, you’re not one of us” . . the way to get buy-in from surgeons is you got to have a surgeon on your team.
A quality manager who agreed to participate in a two-day quality collaborative was reprimanded by the chief nurse—who Saint et al. label as an organizational constipator—for her involvement:
When [the chief nurse] found out I went to the first day, I was told I wasn’t allowed to go to the second day . . no one else could understand why . . there was a control issue.
Table 10-1 chronicles some of the major themes from this study and best practices for overcoming both sources of resistance.
|
It is beyond the scope of this book to cover all aspects of HAIs in great detail; the interested reader is referred to other resources.6–8 Instead, this chapter will highlight some of the patient safety principles involved in preventing the most common HAIs, ending with a discussion of what the patient safety movement can learn from the more established field of infection prevention. Table 10-2 summarizes some of the key evidence-based practices for the prevention of surgical site infections, VAP, CLABSI, and catheter-associated urinary tract infection.6,9 (Note that hand hygiene is an essential practice for the prevention of all HAIs.)
| Healthcare-Associated Infection | Preventive Measure | Definition or Comment |
|---|---|---|
| All healthcare-associated infections | Hand hygiene | Cleaning hands before and after each patient contact |
| Surgical site infection | Appropriate use of perioperative antibiotics | Administration of appropriate prophylactic antibiotic, generally begun within 1 hour of skin incision and discontinued within 24 hours |
| Avoidance of shaving the operative site | Use clippers or other methods for hair removal in the area of incision(s) | |
| Perioperative normothermia | Recommended by some guidelines but controversial | |
| Glucose control | Previous recommendation for tight glucose control has been relaxed after trials showed harm; aim for less than 180 mg/dL | |
| Ventilator-associated pneumonia | Semirecumbent positioning | Elevation of the head of the bed to more than 30° for all mechanically ventilated patients |
| Daily assessment of readiness for weaning | Minimize duration of mechanical ventilation by limiting sedative administration and/or using protocolized weaning | |
| Regular antiseptic oral care | At least every shift | |
| Draining subglottic secretions/silver-coated endotracheal tube | Recommended by some guidelines but controversial | |
| Central line–associated bloodstream infection | Maximum barrier precautions | Use aseptic technique, including cap, mask, sterile gown, sterile gloves, and a large sterile sheet for the insertion of all central venous catheters |
| Chlorhexidine skin antisepsis | Use 2% chlorhexidine gluconate solution for skin sterilization at the insertion site | |
| Appropriate insertion site selection | Subclavian vein is preferred site for nontunneled catheters; avoid femoral site for nonemergency insertions | |
| Prompt removal of unnecessary catheters | Remove when no longer essential for care | |
| Catheter-associated urinary tract infection | Aseptic insertion and catheter care | Use of skin antisepsis at insertion and proper technique for maintenance of catheter and drainage bag; use of closed drainage system |
| Prompt removal of unnecessary catheters | Remove catheter when no longer essential for care | |
| Silver- or antimicrobial-coated catheters | Recommended by some guidelines but controversial |
Surgical Site Infections
Surgical site infections are among the most common adverse events in hospitalized patients,10 and they cause substantial increases in mortality, readmission rates, and costs. Approximately 1 in 30 “clean” surgeries will be complicated by a surgical site infection. The rate is significantly higher for “dirty” (i.e., after trauma), emergency, or prolonged surgeries, and for patients with medical comorbidities.
Guidelines produced as recently as 20086,9 recommend the following strategies to prevent surgical site infections: appropriate use of prophylactic antibiotics (giving guideline-endorsed antibiotics within an hour of the incision, and stopping it/them within 24 hours after surgery), use of clippers (rather than razors) for hair removal prior to surgery, maintenance of postoperative normothermia,11 and maintenance of relatively tight glucose control in the postoperative period.
That final recommendation was driven largely by a prominent 2001 study, which led to great enthusiasm for perioperative intensive insulin therapy.12 Unfortunately, subsequent studies found no evidence of benefit and striking evidence of harm, mostly in the form of frequent hypoglycemic episodes.13,14 As of 2012, therefore, tight glucose control to prevent surgical site infections (or for any perioperative indication) was no longer recommended. Most authorities now recommend keeping glucose levels at less than 180 mg/dL, but not to aim for tight control.15
Ventilator-Associated Pneumonia
Of all HAIs, VAP is the leading cause of death, with far more fatal cases of VAP than of nosocomial urinary tract infections, CLABSI, or surgical site infections.16 About 15% of patients receiving mechanical ventilation, particularly for long periods, will develop VAP, which in turn results in prolonged mechanical ventilation and longer hospital stays. Ventilated patients who develop VAP have a significantly higher mortality rate (46%) than those who don’t (32%).
Many cases of VAP can be prevented by strict adherence to a variety of preventive strategies. The first is elevation of the head of the bed to semirecumbent (at least 30°) position, which in one trial resulted in an 18% decrease in VAP cases.17 Head-of-bed (HOB) elevation decreases the risk of microaspiration of gastrointestinal tract and upper respiratory tract secretions into the lungs, which can serve as a nidus for infection. Although this intervention might seem like a relatively simple undertaking, experience has demonstrated that it can be a challenge, usually because of changes in patient position.18 Several tactics have been recommended to increase adherence with HOB elevation, including enlisting the support of respiratory therapists and family members and placing a visual cue (such as a line on the wall) to help identify whether the bed is in the correct position. Some modern ICU beds come with a built-in tool to provide continuous monitoring of the angle of incline.6
A second effective strategy is daily interruption of sedation for ventilated patients.19 Under one popular protocol, patients’ sedative infusions are stopped each day, allowing patients to “lighten” to the point that they can answer simple questions. This strategy, when coupled with a program of systematic assessment (usually by trained respiratory therapists) regarding readiness for weaning, results in shorter duration of mechanical ventilation, presumably lowering the risk of VAP.20
Several other strategies have been included in various “VAP bundles.” Regular antiseptic oral care has been proven to reduce the incidence of VAP.21 Several systematic reviews have found that continuous drainage of subglottic secretions can lower VAP rates, but the procedure is technically difficult and relatively few institutions utilize it.22,23 Finally, recent evidence has supported the use of silver-coated endotracheal tubes to prevent biofilm generation and ultimately VAP, although the cost-effectiveness of this strategy is still being debated and it is not standard practice as of this writing.24
VAP, like the other HAIs, has been considered for inclusion in a variety of public reporting and payment policies. One challenge, though, is that there are several VAP definitions, which means that the determination of whether an individual patient has VAP may be subject to interpretation. This, of course, opens the door to the possibility that hospitals that use conservative definitions or vigorously monitor their patients for infections may unfairly appear to be unsafe.25,26