Gelatinous Transformation and Other Bone Marrow Stromal Disorders
Jacob Sramek, MD
Key Facts
Terminology
Distinctive patterns of stromal alterations often associated with specific bone marrow insults
Microscopic Pathology
Gelatinous transformation
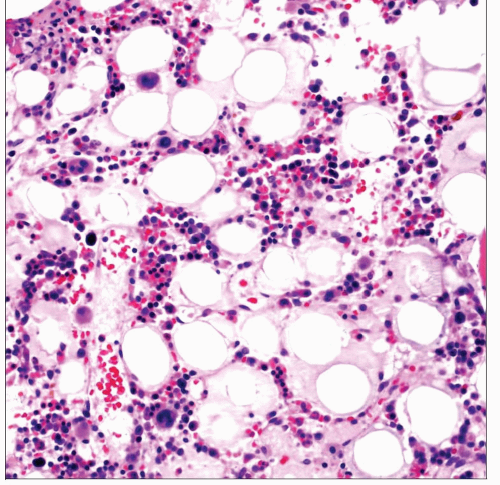
Homogeneous eosinophilic stroma admixed with atrophic adipocytes and decreased hematopoietic elements
Fibrosis
Subtle to marked increase in reticulin fibers best visualized by silver stains
Severe fibrosis is associated with collagen fibrosis (positive by trichrome stain)
Ischemic necrosis
Necrosis of medullary cavity alone or with bony trabeculae
Sections show granular matrix with ghost cells
Fibrinoid necrosis
Eosinophilic granular stroma with net-like appearance seen following chemotherapy
Amyloid deposition
Sections show homogeneous eosinophilic deposits in vessel walls and, less commonly, interstitium
Aspirate smears show homogeneous eosinophilic to dark purple waxy globules
Diagnostic Checklist
Characteristics of stroma and associated cellular, vascular, and bone changes
Histochemical stains
Correlation with clinical history is essential
Many stromal changes can be associated with neoplastic conditions
 This aspirate smear from a patient with AIDS demonstrates extensive gelatinous transformation. The matrix is smooth, uniform, and varies from eosinophilic to pale blue by Wright-Giemsa stain. |
TERMINOLOGY
Synonyms
Gelatinous transformation
Serous fat atrophy
Gelatinous atrophy
Gelatinous degeneration
Starvation bone marrow
Definitions
Distinctive patterns of stromal alterations often associated with specific bone marrow insults
ETIOLOGY/PATHOGENESIS
Gelatinous Transformation
Altered stroma composed of hyaluronic acid with increased glycosaminoglycans
Associated conditions
Malignancy (both hematolymphoid and metastatic)
Malnutrition (including alcoholism and anorexia nervosa)
Infections (particularly AIDS)
Maldigestive conditions
Heart failure
Metabolic disorders
Fibrinoid Necrosis
Seen following myeloablative chemotherapy
Ischemic (Coagulative) Necrosis
Secondary to vascular insufficiency
Associated conditions
Malignancy (particularly B-acute lymphoblastic leukemia)
Sickle cell anemia
Disseminated infections
Anti-phospholipid antibody syndrome
Disseminated intravascular coagulation (DIC)
Fibrosis
Reticulin fibers are normal component of bone marrow stroma typically associated with sinuses, large vessels, and lymphoid aggregates
Increased reticulin fibers and any detectable collagen fibers are pathologic
Induced by multiple factors, including: Transforming growth factor β, platelet-derived growth factor, interleukin-1 (IL-1), substance P, basic fibroblast growth factor, epidermal growth factor, and vascular endothelial growth factor
Some of these factors may be released by apoptotic megakaryocytes and may contribute to fibrosis associated with high megakaryocyte turnover
Amyloid
Deposition of abnormally folded proteins with high β-pleated sheet content
Associated conditions
Primary systemic (AL) amyloidosis
Plasma cell myeloma
Waldenström macroglobulinemia(WM)/lymphoplasmacytic lymphoma
Chronic inflammatory states (AA amyloidosis)
CLINICAL ISSUES
Epidemiology
Incidence
Epidemiology of each of these stromal changes varies based on underlying cause
Amyloid
Systemic amyloidosis is found in up to 15% of patients with plasma cell myeloma
Bone marrow amyloid can be detected in many cases of plasma cell myeloma without overt clinical amyloidosis
Ischemic necrosis is identified in approximately 2% of bone marrow biopsies
Presentation
Ischemic bone marrow necrosis commonly manifests with
Bone pain
Fever, fatigue, jaundice
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree