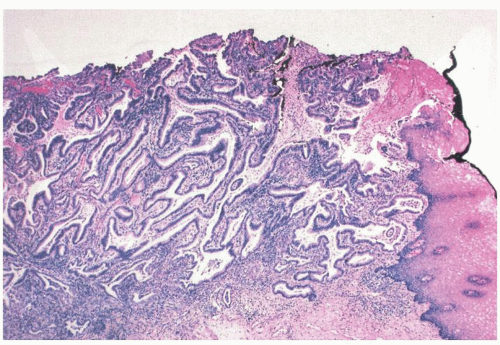
Gastric Epithelial Polyps and Tumors
ADENOMA/DYSPLASIA/INTRAEPITHELIAL NEOPLASIA
Definitions and Terminology
Gastric epithelial neoplastic lesions, which do not fit the criteria for an adenocarcinoma, have traditionally been referred to as adenomas when they are raised, elevated, nodular, or overtly polypoid, or dysplasia when they involve flat mucosa. On the other hand, particularly in Japan, the concept of adenoma/dysplasia is considered as a single entity (adenoma) based on the notion that the cytologic features of adenoma and dysplasia are the same, whether they are elevated, flat, or depressed. The concept of intraepithelial neoplasia (IEN) (low-grade and high-grade) merges these concepts, but has the disadvantage that, in the gastrointestinal (GI) tract, many apparent benign tumors have abnormalities in genes traditionally associated with neoplasia, including some overt syndromes known to be complicated by neoplasia (juvenile polyposis syndrome [JPS], Peutz-Jeghers syndrome [PJS]). Consequentially, the term “intraepithelial neoplasia” can also be viewed conceptually in a broader sense for lesions that have features of a neoplasm but are not (yet) overtly dysplastic, as well as a synonym for dysplastic lesions.1 When used for the latter, like dysplasia, it refers to gastric epithelial neoplastic lesions without lamina propria invasion, irrespective of their gross appearance (elevated, flat, and depressed). From a practical viewpoint, adenoma/dysplasia/IEN is a subjective interpretation of a series of morphologic changes when compared to the normal or metaplastic gastric mucosa.
In this section, we use the following definitions.1
Adenoma—a circumscribed benign neoplasm composed of tubular or villous structures lined by dysplastic epithelium whether elevated, flat, or depressed. It represents interpretation of a series of morphologic changes when compared to the normal or metaplastic gastric mucosa.
Dysplasia is used as the defining characteristic of adenomas. However, it is also used generically for lesions found incidentally on biopsy in which no endoscopic lesion was identified, usually in biopsies showing intestinal metaplasia, fully acknowledging that this may well be dependent on the generation of endoscope used, its resolution, the availability of high-definition monitors, the expertise of the endoscopist together with the care and time taken looking for lesions, and whether tools for accentuating lesions such as narrowband imaging or chromoendoscopy are used.
Dysplasia is subjectively graded into low-grade dysplasia (LGD) and high-grade dysplasia (HGD), the
latter having either loss of polarity or architectural distortion in the absence of invasion in the lamina propria (some may call this carcinoma in situ; it also conforms to what is included as “cancer” in Japan).
latter having either loss of polarity or architectural distortion in the absence of invasion in the lamina propria (some may call this carcinoma in situ; it also conforms to what is included as “cancer” in Japan).
Intramucosal carcinoma has invasion into the lamina propria and is also included in the Japanese term “cancer.”
Invasive carcinoma in the stomach refers to any tumor that has invaded into the lamina propria or beyond.
The major problems in dealing with these are as follows:
1. The distinction of dysplasia/IEN from atypical regenerative and hyperplastic features.
2. Grading when and if this is necessary.
3. The distinction of high-grade lesions from minimally invasive (intramucosal) adenocarcinoma.
Special Stains for Characterizing Gastrointestinal Epithelial cells
The importance of analyzing phenotypic expression of tumor cells using cell lineage markers has been demonstrated in the GI tract.2 The GI mucosa is covered by mucin-producing cells that produce cell-specific mucins. Mucins are highly glycosylated glycoproteins and their core proteins (mucin core proteins: MUC) have been named according to the corresponding genes (mucin gene).3 MUC5AC (sometimes abbreviated simply to MUC5), MUC6, and MUC2 are expressed in gastric surface mucous cells, gastric gland mucous cells (cardiac gland cells, mucous neck cells, and pyloric gland cells), and intestinal goblet cells, respectively.4, 5
Histochemically, gastric mucous cells have neutral mucins (glycoproteins) and show strong reactivity with periodic acid-Schiff (PAS) reaction—usually carried out with diastase to remove any glycogen present—diastase PAS, or D-PAS. On the other hand, intestinal mucous cells, and intestinal metaplasia in the stomach, have acid mucins and show reactivity with Alcian blue (AB) stain or high iron diamine stain for specifically demonstrating acid sialomucins and sulfated mucins (a subgroup of acid mucins), respectively, and also weakly with PAS. A combined AB/DPAS stain demonstrates them both. AB stain can also be used as a substitute for sulfomucin stain depending on the pH at which the stain is carried out. Staining at pH 2.5 detects acid mucins (both sialomucins and sulfated mucins) but at pH 1.0 it detects sulfated mucins but not sialomucins.
In addition to mucins, CD10, a 100-kD surface metal-loendopeptidase, and other brush border proteins such as villin, is specifically expressed on the brush border of small intestinal epithelial cells in the GI tract.6 The expression of these mucins and intestinal enzyme are cell type specific; hence, they are useful phenotypic indicators of cell differentiation in normal, metaplastic, or neoplastic epithelial cells in the GI tract.2
The endocrine cells can be demonstrated using either specific markers (e.g., gastrin, somatostatin, serotonin, ghrelin) or nonspecific markers (synaptophysin, chromogranin A, CD56, CD57) while vesicular monoamine transporter 2 (VMAT2) stains enterochromaffin-like cells. These are discussed further in Chapters 5 and 12.
Classification of Gastric Adenomas
Architecturally, gastric adenomas are categorized as tubular, tubulovillous, or villous adenoma.1 Tubular adenomas consist of tubules covered by dysplastic epithelium and surrounded by lamina propria. The dysplastic epithelium lining these tubules often remains relatively superficial, but sometimes can extend from the surface to the crypt base. They appear as rounded or oval structures on cross section. Villous adenoma consists of villi with a core of lamina propria covered by dysplastic epithelium and often shows a papillary configuration with branching. Tubulovillous adenoma has a mixture of tubular and villous structures, each contributing at least 25% to the tumor.1
Phenotypically, gastric adenoma can be classified as follows:
1. Intestinal-type adenoma
2. Gastric-type adenoma
i. Pyloric gland adenoma
ii. Foveolar adenoma
iii. Fundic gland adenoma
In practice, it is probably more accurate to think of intestinal, foveolar, and pyloric gland dysplasia, so that if they occur in association with other polyps/lesions they are recognized. The majority of gastric adenomas belong to the intestinal type, and gastric-type adenomas are less common, but not rare, although foveolar adenomas far outnumber pyloric gland adenomas. There are clinical and morphologic differences between intestinal-type and gastric-type adenoma.
Intestinal-type adenomas. Intestinal-type adenomas are composed of varying admixtures of goblet cells, intestinal-type absorptive cells, Paneth cells, and occasional endocrine cells, or columnar cells with varying degrees of differentiation.
Clinical Features Intestinal-type adenomas occur predominantly in the sixth to seventh decades of life, more frequently in males than in females (3:1) and are associated with chronic gastritis with atrophy and intestinal metaplasia7 and often also with Helicobacter pylori infection in the nonmetaplastic mucosa.8 They
tend to arise more frequently in the lesser curvature of the antrum and angulus.
tend to arise more frequently in the lesser curvature of the antrum and angulus.
The frequency of carcinoma arising in adenomas depends on tumor size and histologic grade. In the study by Kamiya et al.,9 85 mostly small adenomas were followed for up to 12 years. During the follow-up, many adenomas grew little and sometimes even diminished in size; however, focal carcinomas (carcinoma in situ) were detected in 11% (nine lesions) and seven invasive carcinomas developed in those more than 2 cm in diameter.9 Increasing size of the adenoma was helpful in predicting the development of carcinoma in these lesions.9 Saraga et al.10 found that low-grade adenomas remained stable for many years, whereas high-grade adenomas were frequently associated with cancer. Another study from Italy showed that about 90% of adenomas with LGD did not become invasive carcinoma in 5 years, whereas 80% with HGD did.11
Clinical implications: It is usually best to remove all adenomas endoscopically, by polypectomy, endoscopic mucosal resection (EMR), or endoscopic submucosal dissection (ESD). Alternatively, while low-grade adenoma should ideally be removed endoscopically, if impractical, patients require at least subsequent endoscopic checkups since the lesion may evolve into an invasive cancer. This is the case when there are multiple adenomas and all cannot be definitively removed. Hence the endoscopist usually focuses on the largest lesions with the rationale being the correlation between size and malignant potential. High-risk adenomas (larger than 2 cm in diameter,9 high-grade adenomas10, 11, 12) should be removed endoscopically unless there are good contraindications.
Pathologic Findings In the elevated (sessile polypoid) form, typically adenomatous epithelium is confined to the upper to mid mucosa overlying nonneoplastic mucosa with normal or more often cystically dilated glands. In flat and depressed adenomas, the adenomatous epithelium occupies almost the entire thickness of the mucosa.
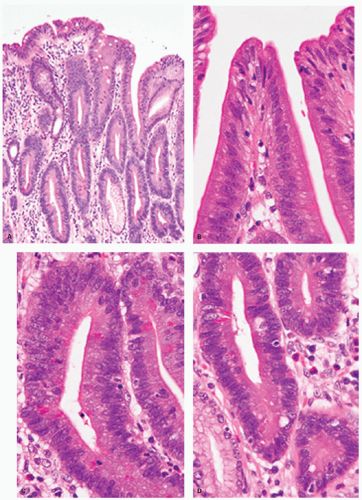
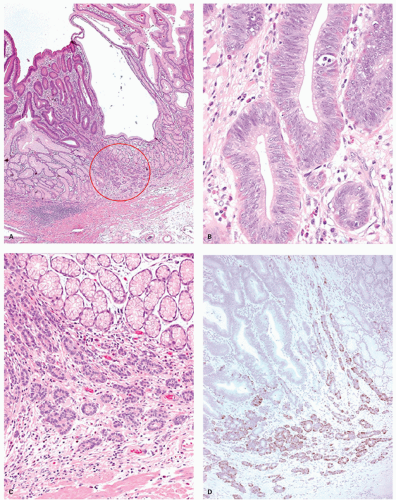
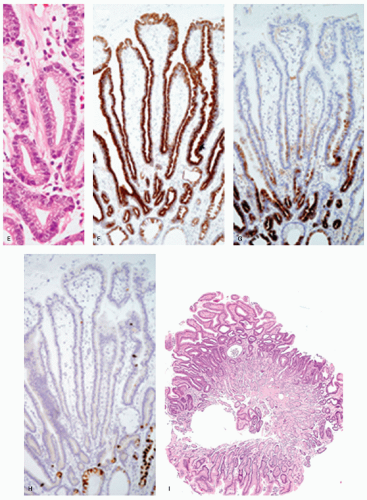
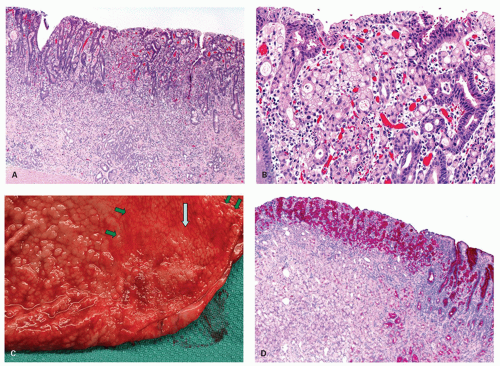
Intestinal-type adenomas can be subdivided into exclusively intestinal phenotype (complete intestinal type) (Fig. 14-1) and mixed gastric and intestinal phenotype (incomplete intestinal type) (Fig. 14-2) using immunostaining for GI cell lineage markers (see below).13, 14 The majority of low-grade adenomas are the complete intestinal type and this type seems to be stable.14 On the other hand, mixed gastric and intestinal phenotype adenomas predominate over the complete intestinal type in high-grade adenomas and also in intramucosal carcinomas.14 In addition, a long-term follow-up study revealed that adenomas that developed into adenocarcinoma frequently displayed gastric-type differentiation (incomplete metaplasia).15 Thus a gastric phenotype in gastric adenoma suggests higher malignant potential.
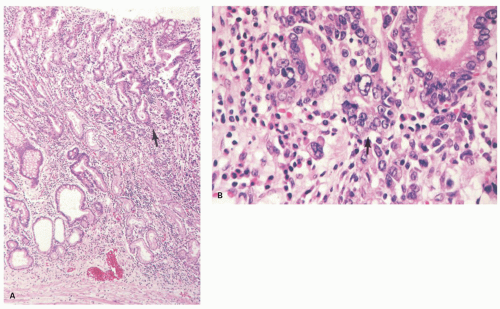
Histologically, dysplastic glands of complete intestinal-type adenomas tend to be straight and are lined by columnar cells with acidophilic cytoplasm similar to intestinal absorptive cells with scattered goblet cells and sometimes Paneth cells near the mid-part or the base of the dysplastic glands (Fig. 14.1A-D). Superficially, nuclei are elongated and the nuclei are less stratified (Fig. 14-1B). In the deeper part of the glands the nuclei tend to be larger with more stratification and scattered mitoses, seemingly forming a generative zone (Fig. 14-1C). Toward the base, the dysplastic cells have basally oriented, enlarged nuclei containing tiny nucleoli (Fig. 14-1D).
Dysplastic glands of the mixed gastric and intestinal-type adenoma show more architectural distortion with irregular crypts lined by goblet cell-type cells, columnar mucous cells showing variable stages of differentiation, and less frequent Paneth cells (Fig. 14.2A-C).
Rarely, in atrophic stomachs, intestinal-type carcinoid tumors arise in gastric tubular adenoma (Fig. 14-3).16, 17
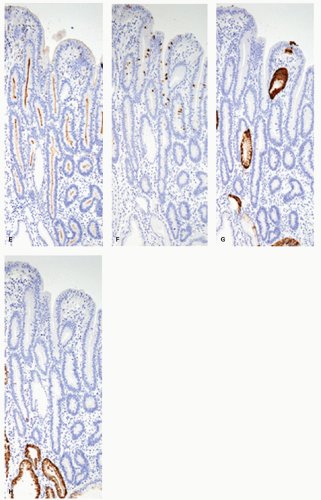
Immunohistochemically, in complete intestinal-type adenoma, CD10 is expressed on the luminal surface of intestinal absorptive-type cells (Fig. 14-1E), MUC2 (Fig. 14-1F) is expressed in the goblet cells, and MUC5AC (Fig. 14-1G) and MUC6 (Fig. 14-1H) are negative, resembling the phenotype of complete intestinal metaplasia or small intestinal epithelium. MUC2-positive cells tend to be distributed more superficially (Fig. 14-1F). Ki-67-positive proliferating cells are densely distributed beneath the superficial layer, the generative zone. In mixed gastric and intestinal-type adenoma, resembling phenotype of incomplete intestinal metaplasia, goblet cells and columnar cells show varying degrees of reactivity for MUC5AC (Fig. 14-2D), MUC6 (Fig. 14-2E), and MUC2 (Fig. 14-2F), but not for CD10 (Fig. 14-2G). MUC5AC-positive cells, MUC2-positive cells, and MUC6-positive cells tend to be distributed in the upper or deeper layers, respectively (Fig. 14-2D-F). The Ki-67-positive proliferating cells are more concentrated in the deeper parts.
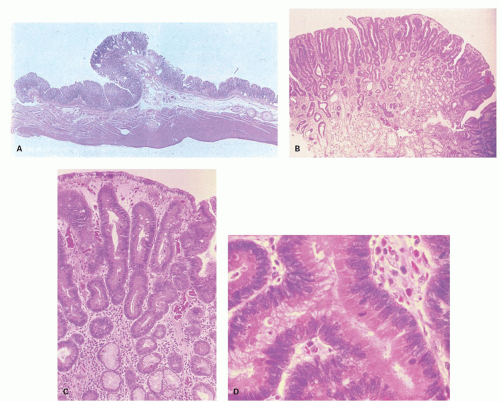
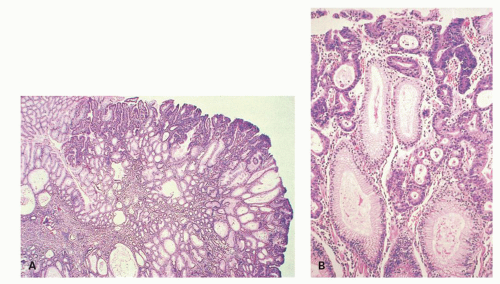
Gastric-type adenomas. Gastric-type adenomas are composed of cells native to normal gastric mucosa, and there are two subtypes. Pyloric gland adenomas predominantly consist of pyloric gland mucous cells (Fig. 14-4), whereas foveolar adenomas are predominantly composed of mucous cells resembling the mucous cells of the gastric surface epithelium and foveolae (Fig. 14-5). By definition, both are dysplastic, although the nuclear atypia often tends to be quite subtle. Dysplasia arising in other polyps (hyperplastic, fundic gland, hamartomas) tends to be of foveolar or intestinal type if intestinal metaplasia is present.
Pyloric gland adenoma. The phenotypic characteristics of pyloric gland adenoma were first described by Borchard et al.18 and by Watanabe et al.19 in 1990. Since then, Vieth et al.20 and Kushima et al.21 reported a more detailed clinicopathologic analysis of pyloric gland adenoma.
Clinical Features Pyloric gland adenomas of the stomach are said to account for about 2.7% of all gastric polyps and occur commonly in the elderly, the mean age being around 73 ± 12.8 years, and invariably occur in corpus mucosa (64%). This is a little surprising as one would expect them to arise in the antrum where pyloric glands are normally present. However, while they are mainly associated with atrophic gastritis and gastric atrophy, they can be found anywhere between the lower esophagus and duodenum, gallbladder, and presumably wherever there is pyloric metaplasia. The male-to-female ratio is 1:3. In the European population, they are often found in patients with autoimmune gastritis (36%).20
Pathologic Findings Macroscopically, pyloric gland adenomas usually present as nodular and dome-like lesions primarily in the oxyntic mucosa, although there is no reason why they should not occur wherever gastric-type mucosa is found.22, 23
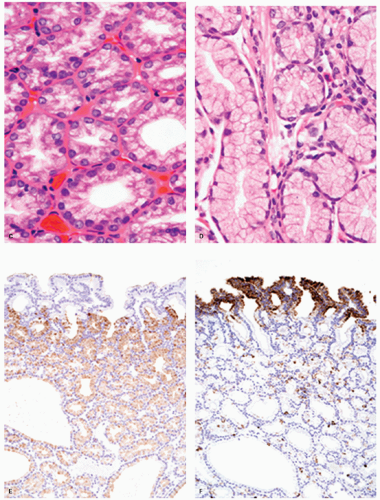
Microscopically, they are principally composed of lobules containing closely packed pyloric-type glands lined by columnar or cuboidal epithelial cells connected to gastric foveola-like structures confined superficially (Fig. 14-4A). These glandular cells show clear or pale eosinophilic cytoplasm and small and oval to round-shaped nuclei with small but conspicuous nucleoli (Fig. 14-4B,D).21 However, the changes are quite subtle, and unless one deliberately compares normal pyloric gland nuclei with those seen in these lesions and looks for the open chromatin and small nucleoli they are easy to miss. In one study, adenocarcinoma was found in 30% of gastric pyloric adenomas and a possibility of pyloric gland adenoma-adenocarcinoma sequence has been raised.21 Although these adenomas can clearly be seen to infiltrate adjacent tissue, their metastasizing potential is still unclear. This creates a problem when these extend to polypectomy margins and complete removal entails major resections, for example, duodenal pyloric gland adenomas abutting onto pancreas (see subsequent section on pyloric gland carcinomas).
Immunohistochemically, most glandular cells are positive for MUC6 (gastric pyloric/antral gland mucous cell marker) (Fig. 14-4E); on the other hand, cells of the foveolar-like structure and some glandular cells are positive for MUC5AC (gastric surface and foveolar cell marker) (Fig. 14-4F).21 Considering that MUC5AC is expressed in mucous cells of gastric epithelium in fetal gastric mucosa,24 colocalization of MUC5AC and MUC6 in cells of gastric pyloric gland adenoma suggests an immature phenotype of these cells. MUC2 and CD10 are generally negative.21 These tumors are often considered to be tumors with a predominantly mucous neck cell phenotype. The Ki-67-positive proliferating cells are distributed in the deeper parts of the foveolar structures and in the superficial parts of pyloric-type glands.
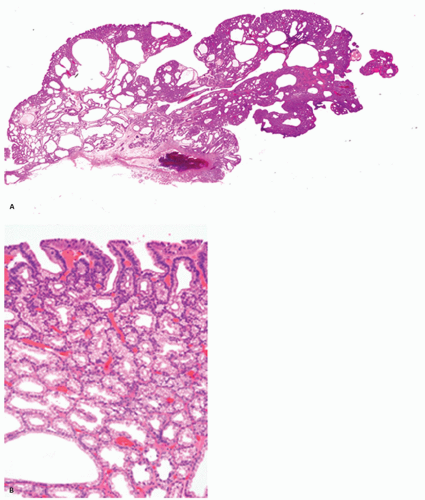
Foveolar adenoma. Foveolar adenomas are composed predominantly of dysplastic surface or foveolar-type cells with an apical cap of neutral mucin. They express gastric surface mucous cell-type mucin (MUC5AC) but not intestinal mucin.25, 26, 27a, 27b, 27c There is an increased incidence of foveolar adenoma in patients with familial adenomatous polyposis.25 Sporadic foveolar adenomas are uncommon, but not rare.
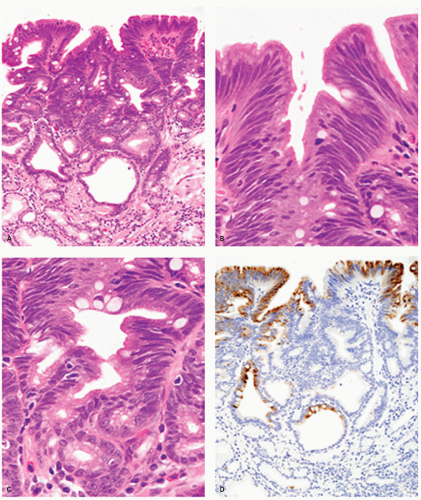
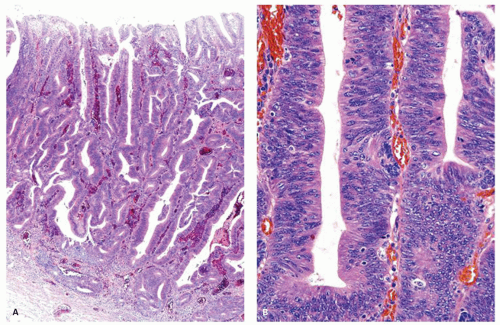
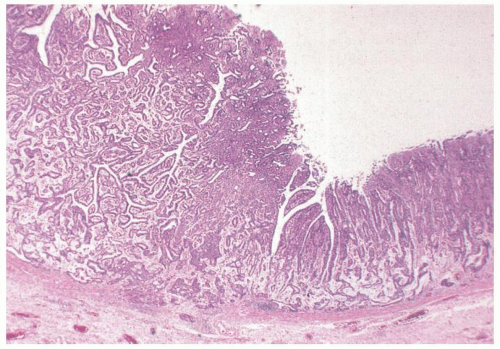
Pathologic Findings This type of adenoma may have a papillary/villous structure (Fig. 14-5). On biopsy specimens, tumors showing papillary/villous structure composed of columnar or cuboidal cells with clear cytoplasm rich in neutral mucins are often difficult to differentiate from well-differentiated adenocarcinoma of gastric type.21
Adenocarcinoma of gastric foveolar type are superficially very similar, often having papillary/villous projections in the upper portion but also irregular branching/fusion (Fig. 14-6) and an intramucosal carcinoma in the middle to deeper portion.21 In the foveolae of both, adenoma and the well-differentiated adenocarcinoma of gastric type, the upper portions of the papillary/villous structures are lined by tall columnar cells with enlarged cell size and low nuclear-cytoplasmic ratio (Figs. 14-5A-C and 14-6A,B) and resemble the surface and foveolar epithelium (Fig. 14-5D), whereas the deeper portions are lined by cuboidal cells resembling pyloric gland cells (Fig. 14-5E). These cells have varying degrees of mucinous cytoplasm and basally situated nuclei, which are slightly larger than those of normal gastric mucosa and are hyperchromatic.
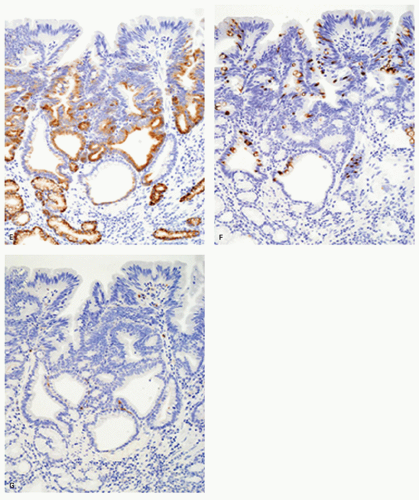
Immunohistochemically, foveolar adenoma and well-differentiated adenocarcinoma of gastric type show similar mucin phenotypes. They show immunoreactivity for MUC5AC (Fig. 14-5F) and MUC6 (Fig. 14-5G) in the upper and lower portions of the papillary structures, respectively, showing differentiation similar to gastric pyloric mucosa. MUC2-reactive cells may be only focal or sporadic, if present (Fig. 14-5H), and CD10 is generally negative. The Ki-67-positive proliferating cells are distributed in the lower parts of the foveolar structures and in the upper parts of pyloric-type glands.
Fundic gland adenoma. We include this section cautiously as these are still poorly understood and characterized. Lesions are encountered such as those seen in fundic gland carcinoma (see subsequently), but without any evidence of submucosal invasion. Some of such lesions encountered consist of multilayered fundic gland epithelium that have been described
as chief cell adenomas, parietal cell adenomas, and also as mucous neck gland adenomas. Some consider the chief cell predominant type as an intermediate phenotype between mucous neck cell and chief cell (immature chief cell).27a, 27b, 27c The lesions are well circumscribed and appear to be able to differentiate along both parietal and chief cell lineages, and the term used depends on the dominant cell type, unless one uses an all inclusive term such as fundic gland (or mucous neck) adenoma. Because of their similarity to fundic gland carcinomas they are illustrated with that section to allow easy comparison in Figure 14-27. These lesions are rare but clearly different from fundic gland polyps, and their natural history is unknown.
as chief cell adenomas, parietal cell adenomas, and also as mucous neck gland adenomas. Some consider the chief cell predominant type as an intermediate phenotype between mucous neck cell and chief cell (immature chief cell).27a, 27b, 27c The lesions are well circumscribed and appear to be able to differentiate along both parietal and chief cell lineages, and the term used depends on the dominant cell type, unless one uses an all inclusive term such as fundic gland (or mucous neck) adenoma. Because of their similarity to fundic gland carcinomas they are illustrated with that section to allow easy comparison in Figure 14-27. These lesions are rare but clearly different from fundic gland polyps, and their natural history is unknown.
Grading of Adenoma/Dysplasia/Intraepithelial Neoplasia
Adenomas can be subjectively graded into low grade and high grade; indeed, there are numerous classifications of dysplasia/intraepithelial (noninvasive) neoplasia, but the two-grade (high and low grade) system is most consistent.28, 29, 30, 31, 32 There is no real utility in using an older three-grade system (mild, moderate, severe dysplasia), as the current literature even for other sites, including other parts of the GI tract, uses a two-grade system, so there is no “action” plan that addresses a three-grade system. Also, more objective criteria can be applied to a two-grade system, which should therefore result in greater reproducibility. Nevertheless, it has to be appreciated that this is a continuum of changes as well as focality within the lesion (we grade the highest), so that in the midrange there will inevitably be less reproducibility. However, as all adenomas need to be removed endoscopically where possible, and, surprisingly, in practice most adenomas seem to classify fairly readily, this tends to be less of an issue than might be expected.
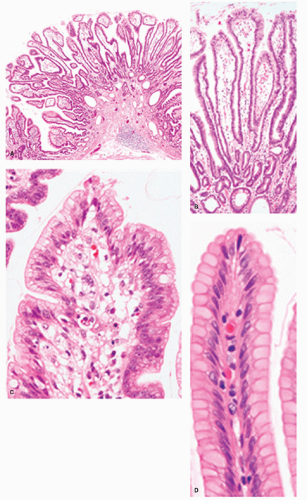
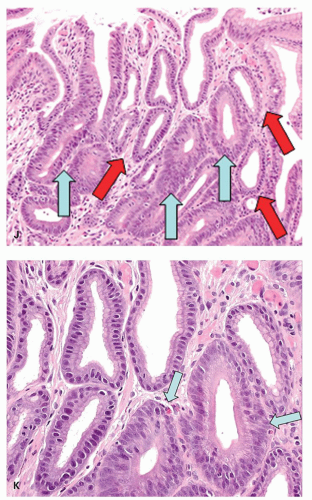
Low-grade adenoma/dysplasia/intraepithelial neoplasia. In intestinal-type adenomas, most cells have deeply staining eosinophilic or amphophilic columnar cytoplasm but goblet cells are common, particularly in lower grades of dysplasia. Enterochromaffin cells and, less frequently, Paneth cells may be present. Tumor cells have closely packed elongated nuclei with dense chromatin. If they show nuclear pseudostratification, the nuclei retain their polarity and they are confined to the basal half or two-third of the cell (Fig. 14-7). In low-grade adenomas, mitoses are usually limited to the superficial third of the mucosa.
High-grade adenoma/dysplasia/intraepithelial neoplasia. High-grade adenomas are graded primarily in intestinal epithelium, where it has more marked architectural and cytologic atypia, but having “more” changes states overtly that this is a subjective evaluation, and where LGD stops and HGD starts is subjective. The ends of the spectrum have relatively little interobserver variability when both are classical in either the metaplastic or the foveolar pathway. The architectural changes produce crowded glands that may be irregularly shaped with frequent branching and budding. In extreme cases, cribriforming may also be found.
Cytologically, high-grade adenoma consists of two main types. In the intestinalized variant, nuclei may be elongated and hyperchromatic and of irregular size. In this type, nuclear stratification may regularly extend to the cell surface, failing to mature into recognizable cell types, and nuclear polarity may be lost (Fig. 14-8). In foveolar dysplasia, tumor cells often are paler and cuboidal rather than having elongated cytoplasm and have ovoid to round and vesicular nuclei with irregular contours and prominent nucleoli. In these cases, nuclear stratification may be less prominent than in low-grade adenoma, but frequently with loss of polarity (an observation that, in Japan, is included as diagnostic criteria of “carcinoma”) (Fig. 14-9). Mitoses are often numerous, and atypical mitoses may be noted. In foveolar dysplasia, the grading of dysplasia can be very difficult as the nuclei may be basal (low-grade) but the cytological detail high-grade, with loss of polarity that is again very subjective. Fortunately as they all need to be removed
endoscopically (if possible), the distinction matters less when treated this way.
endoscopically (if possible), the distinction matters less when treated this way.
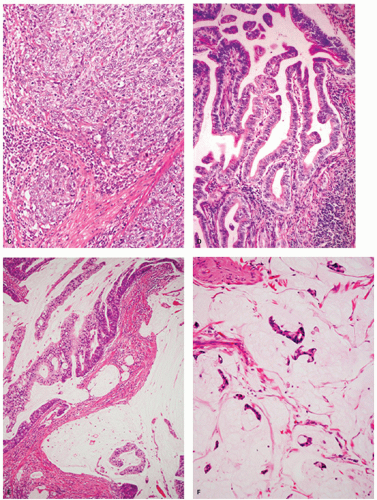
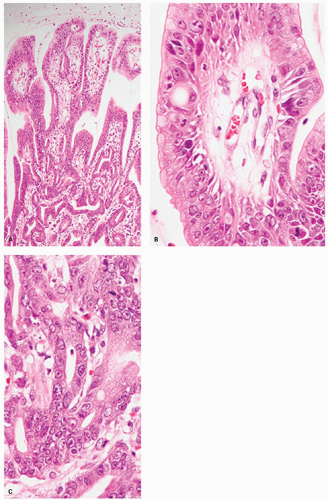 Figure 14-6. Well-differentiated gastric-type adenocarcinoma. A: Well-differentiated gastric-type adenocarcinoma showing papillary projections in the upper portion and irregular branching/fusion in the deeper portion. B: A papillary projection is lined by columnar cells with clear staining cytoplasm and basally oriented enlarged vesicular nuclei with prominent nucleoli. In contrast, the nuclei of normal foveolar epithelial cells have evenly distributed chromatin with inconspicuous nucleoli in Figure 14-5D. In the deeper portion, tumor shows irregular fusion indicative of invasion of the lamina propria (C). |
Differential Diagnosis
Distinction of regenerative changes from adenoma (dysplasia/intraepithelial neoplasia). Reparative changes in the stomach are common. Most occur in native foveolar mucosa, but variants include those arising in intestinal metaplasia, both complete and incomplete, and each is different morphologically. The latter can be recognized by the presence of the metaplasia in the adjacent mucosa, or goblet cells within the regenerating mucosa. However, complete intestinal metaplasia starts with intestinal nuclei that are already considerably larger than native mucosa, so this needs to be taken into account, while nuclei in incomplete intestinal metaplasia are more open and vesicular with distinct small nucleoli. Reactive changes therefore enhance all of these features. All forms of reactive mucosa have “acute” mucin depletion and enlarged pleomorphic nuclei occupying most of the cell (see Chapter 13 and Fig. 14-5K), and may be accompanied by erosions or ulcers. The tip-off is the presence of the following features:
1. Restituting (regenerating) mucosa (low cuboidal or columnar) with nuclei that are usually more widely separated than in normal mucosa, especially superficially. These may be totally devoid of mucin and
nuclei make up virtually the entire cell. Mitoses may be present on the surface and high in the pits. Vesicular nuclei with a thin nuclear membrane and prominent nucleoli may be present. Adjacent nuclei may vary in size and shape, but do not have the marked uniform hyperchromatism, the heavy chromatin rim, or the marked overlapping that characterizes dysplasia, although an occasional uniformly hyperchromatic nucleus may be seen.
nuclei make up virtually the entire cell. Mitoses may be present on the surface and high in the pits. Vesicular nuclei with a thin nuclear membrane and prominent nucleoli may be present. Adjacent nuclei may vary in size and shape, but do not have the marked uniform hyperchromatism, the heavy chromatin rim, or the marked overlapping that characterizes dysplasia, although an occasional uniformly hyperchromatic nucleus may be seen.
2. Usually a degree of maturation superficially, to the degree that a diagnosis of dysplasia should be made very cautiously in the presence of active restitution. Cytoplasm may remain very eosinophilic.
3. In the bases of these pits nuclei can overlap and be stratified and hyperchromatic, and cause problems and are therefore likely to be confused with adenoma.
4. The lamina propria can show a variety of changes from chronic reactive features (capillary dilatation, hypertrophy of smooth muscle, an assortment of inflammatory cells), but sometimes it is densely hyalinized and acellular. This immediately suggests that the lack of inflammation makes Helicobacter unlikely and that the etiology is therefore very likely to be medications (see below).
There are some diagnostic problems. In some cases, especially in polyps, enlarged nuclei that are often stratified are found in which there is little or no ulceration or inflammation; these nuclei therefore cannot be ignored, yet they are not blatantly dysplastic. They can be handled in a variety of ways; the only mandatory procedure is to take multiple levels to ensure that an underlying carcinoma is not lurking elsewhere in the polyp. It is usually best to utilize the terms atypical changes or indefinite for dysplasia, but if the polyps have been removed it is of no clinical significance, and this needs to be stated. Because the regenerative pattern itself may produce a back-to-back appearance, albeit rarely, care must be taken not to add a little nuclear aberration to the architectural abnormality and make a diagnosis of carcinoma in situ.
The causes of reactive changes and its morphology are described in more detail in Chapter 13 (Gastritis) and include proximity to gastric ulcers and erosions, ingestion of medications such as nonsteroidal (NSAID) anti-inflammatory drugs and alcohol, and in the duodenogastric reflux, particularly following a Billroth II anastomosis, H. pylori-associated gastritis, and also shock.
Distinction of high-grade dysplasia/adenoma/intraepithelial neoplasia) from invasive carcinoma. Criteria for the distinction of high-grade adenoma from carcinoma are different between the Western viewpoint and the Japanese viewpoint.33 Because of the abundant lymphatic and capillary supply in the gastric mucosa, infiltration into the lamina propria has a low but definite metastasizing potential. For this reason, infiltration into the lamina propria warrants a diagnosis of intramucosal carcinoma from the Western viewpoint, but is just included as “cancer” from the Japanese (Fig. 14-10),33 which is based on cytologic and architectural criteria and on the assumption that carcinoma cells must be present within an epithelial layer before beginning stromal invasion. The proliferating tubules of infiltrating carcinoma are often smaller than the adjacent dysplastic tubules and may sometimes infiltrate as irregular glands, small clusters of cells with or without lumina, and ultimately single cells. The most difficult cases are those in which there is proliferation of a small number of regular dysplastic tubules budding into the lamina propria. Sometimes these are a little irregular and may have small papillary projections. However, the tubules do share common walls, so that the term gland within gland or back-to-back is applicable. In these cases, it is a subjective decision as to whether the lesion should be called high-grade adenoma (dysplasia/IEN) that includes carcinoma in situ, with or without invasion of the lamina propria. Interestingly, three-dimensional reconstruction study of adenocarcinoma showed that one of the distinctive features of invasive growth of well-differentiated adenocarcinoma into the lamina propria is an anastomosis among the tubules, producing loops, which is responsible for generating patterns resembling X, Y, and H shapes (lateral fusion) on two-dimensional sections.34
GASTRIC CARCINOMA
Introduction
Gastric carcinomas occur in two main parts of the stomach: Cardia and the distal stomach (antrum and
lesser curvature of the body). They occur more frequently in the latter.
lesser curvature of the body). They occur more frequently in the latter.
Gastric Cardia Carcinoma
Carcinoma of the cardia differs clinicopathologically from those of the rest of the stomach (noncardia cancer) and appears to have different predisposing causes from noncardia cancer and some similarities to esophageal adenocarcinomas.35 Contrary to a decline in noncardia cancer, the incidence of cardia cancer is increased, especially in the Western countries.35, 36
This slightly awkward term is used for carcinomas at the gastroesophageal junction, the problem being exactly what that is. Conceptually, this is where the esophagus ends and the stomach begins, but in practice there are no universally accepted reproducible landmarks that identify exactly where this is. In practice it is usually identified endoscopically as the top of the gastric folds, or the lower end of the vascular arcades, although these are not always the same (see chapter on normal stomach). However, when tumors are large it can be impossible to know precisely where they originated and often their epicenter is used to classify them. One popular classification system is the Siewart system in which carcinomas more than 3 cm above the upper end of the gastric folds are Barrett’s associated cancers, those more than 2 cm below (subcardial) are gastric, and the remainder are cardiac. The problem is that as the tumors get smaller those in the subcardiac region are clearly gastric, while those in the cardia that touch the gastroesophageal junction (GEJ) can be either of gastric or of Barrett’s origin (unless a rare histologic type that makes it gastric).
As per the current AJCC classification tumors that are either clearly below the GEJ and not involving it or those that are more than 5 cm below the GEJ when GEJ is involved are considered cardiac. Despite, problems in the classification, they are worth separating because subcardial cancers have a better prognosis. Nevertheless, the morphology is the same as for other gastric carcinomas. When compared with subcardia cancers, cardiac cancers had a higher male-to-female ratio, tended to be elevated, and had a higher incidence of mediastinal node metastasis, a higher tumor, node, metastasis (TNM) stage, and a significantly lower patient survival rate.37, 38 On immunohistochemical staining, loss of p16 and SMAD 4 and overexpressions of carcinoembryonic antigen (CEA) and CD44 were more frequent in cardia carcinoma than in non-cardia carcinoma, as was Epstein-Barr virus (EBV) infection.37 Many tend to lump gastric cardia cancers with distal esophageal adenocarcinomas, however, these also have important differences despite some similarities.
As per the current AJCC classification tumors that are either clearly below the GEJ and not involving it or those that are more than 5 cm below the GEJ when GEJ is involved are considered cardiac. Despite, problems in the classification, they are worth separating because subcardial cancers have a better prognosis. Nevertheless, the morphology is the same as for other gastric carcinomas. When compared with subcardia cancers, cardiac cancers had a higher male-to-female ratio, tended to be elevated, and had a higher incidence of mediastinal node metastasis, a higher tumor, node, metastasis (TNM) stage, and a significantly lower patient survival rate.37, 38 On immunohistochemical staining, loss of p16 and SMAD 4 and overexpressions of carcinoembryonic antigen (CEA) and CD44 were more frequent in cardia carcinoma than in non-cardia carcinoma, as was Epstein-Barr virus (EBV) infection.37 Many tend to lump gastric cardia cancers with distal esophageal adenocarcinomas, however, these also have important differences despite some similarities.
Carcinoma of the Gastric Antrum and Body
The incidence and mortality of gastric cancer have fallen worldwide.39 However, gastric cancer remains the fourth most common cancer and the second leading cause of cancer-related death in the world.40, 41 Its incidence varies greatly from country to country. High-incidence areas include Eastern Asia, East Europe, and Central and South America.41 Low incidence rates are found in North America, Northern Europe, North and East Africa, and Southeastern Asia.41
Gastric carcinomas do not consist of a homogeneous group of tumors. There are several distinct clinicopathologic entities with different predisposing conditions and probably etiologies. They can be divided into two major histologic types: Those arising in native mucosa and those arising in metaplastic (intestinal) mucosa. These were called intestinal or differentiated type (in metaplastic mucosa) and diffuse or undifferentiated type, corresponding to carcinomas arising in native gastric mucosa, although there is an overlap of patterns in some and others defy classification. Intestinal-type carcinomas predominate in high-incidence geographic areas for gastric carcinoma42 and its incidence has decreased.43, 44 Conversely, diffuse-type carcinoma has a more uniform geographic distribution and is relatively more common in low-incidence areas44 and its incidence has been stable43 or has increased.44 To some extent, they appear to have different underlying causes, precursor lesions, and rates of growth. While these fundamental histologic types have stood the test of time generally, more useable classifications are now in place (discussed subsequently).
Pathogenesis
Pathogenetic factors will be discussed in terms of risk factors, predisposing conditions, and premalignant lesions.
Risk Factors
A risk factor is an attribute that confers a higher than expected incidence of cancer on a population. In gastric carcinoma, there are marked geographic variations, possibly due to environmental factors. Genetic predisposition may also be a factor. Epidemiologic studies have clearly shown that migration from a high-risk area (e.g., Japan) to a low-risk region (e.g., Hawaii) is associated with a decline in the incidence of gastric cancer, most notably in the second generation.45, 46, 47 Similar findings have been reported from Colombia.46 These studies have also shown that environmental factors are important in the genesis of carcinoma.
Helicobacter pylori infection. Epidemiologic studies have revealed a strong association of H. pylori infection with intestinal or diffuse type of gastric cancer development.48 However, the absolute risk is only about twice that of the non-H. pylori-infected population, even though H. pylori is designated as a major (Class 1) risk factor. Supporting this, animal models with H. pylori infection showed promotion of gastric carcinogenesis.49, 50, 51 Prospective cohort studies have shown the strongest evidence to support the role of H. pylori infection in gastric cancer development, although the relative risk (RR) is only a little over two. Yet with so many in the world being infected, this is a significant risk on a worldwide basis. In a prospective study of 1,526 Japanese participants, gastric cancers developed in 2.9% of H. pylori-infected people and in none of the H. pylori-noninfected individuals.52 Furthermore, it has been demonstrated that H. pylori eradication reduced the incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer.53 It is involved in both types of carcinoma, although the association with intestinal-type carcinomas appears most marked.
H. pylori is involved in the development of gastric cancer through two major pathways: The indirect action on gastric epithelial cells through inflammation and the direct action on epithelial cells through the induction of protein modulation54, 55 and gene mutation.56 Inflammation is found especially with organisms containing CpG island methylator phenotype (CIMP), responsible for production of CagA and VacA toxins (see next section).
In the indirect pathway of chronic H. pylori gastritis, enhancement of helper Th1-type CD4 T-cell infiltration and their products, interferon gamma, and a variety of proinflammatory cytokines have been
demonstrated and play a crucial role in the induction of gastritis.57, 58, 59 Among proinflammatory cytokines enhanced in H. pylori-related gastritis, both interleukin (IL)-1β and tissue necrosis factor alpha (TNFα) enhance nuclear factor-κB (NF-κB) activation in epithelial, inflammatory, and mesenchymal cells. NF-κB functions as a tumor promoter in inflammation-associated cancer60 and exerts an antiapoptotic action61 with the production of various cytokines61 and cyclooxygenase-2.62
demonstrated and play a crucial role in the induction of gastritis.57, 58, 59 Among proinflammatory cytokines enhanced in H. pylori-related gastritis, both interleukin (IL)-1β and tissue necrosis factor alpha (TNFα) enhance nuclear factor-κB (NF-κB) activation in epithelial, inflammatory, and mesenchymal cells. NF-κB functions as a tumor promoter in inflammation-associated cancer60 and exerts an antiapoptotic action61 with the production of various cytokines61 and cyclooxygenase-2.62
In the direct pathway, H. pylori directly perturbs intracellular signaling events by delivering bacterial agents into the cells through a bacterial type IV secretion apparatus, encoded by the HP cag pathogenicity island (cagPAI).54, 55 H. pylori also directly activates NF-κB in the gastric epithelial cells and induces gene mutations in epithelial cells by enhancing the expression of activation-induced cytidine deaminase (AID) in gastric epithelial cells through NF-κB activation.56 AID, a member of the cytidine deaminase family, is essential for somatic hypermutation and class-switch recombination of immunoglobulin genes in B lymphocytes by acting as an editor of DNA and RNA.63
In addition, H. pylori infection enhances aberrant DNA methylation in the gastric mucosa through direct action of H. pylori on epithelial cells or H. pylori-induced inflammation, and the methylation may cause gastric carcinogenesis by silencing the tumor suppressor gene.64
Epstein-Barr virus, CIMP-H, and K-ras. EBV is a double-stranded DNA virus that is associated with about 10% of gastric carcinomas, yet the data are a little conflicting. Cancers occur predominantly in a slightly younger male population. Nevertheless,
1. Gastric carcinomas with lymphoid stroma65, 66, 67 have a strong association with EBV, and in about 85% of these tumors, by in situ hybridization.67
2. If one looks at an unselected series of gastric carcinomas, about 8% to 10% of those will also contain EBV, and these are fairly equally distributed between intestinal and diffuse types. This suggests either that the lymphoepithelial variant may transform into regular variants or that regular variants are by far the most common EBV-associated carcinomas, but that when the rare lymphoepithelial variant occurs it is much more likely to be EBV associated.
Whether EBV infection of gastric stem cells occurs from the associated lymphoid cells or occurs directly is unclear, but data suggest that the virus is found only in the epithelial cells. Nevertheless, the mechanism of EBV carcinogenesis appears to be through methylation of the promoter region of many cancer-related genes, and subsequent transformation and selection of the predominant clones.67 Interestingly, the CpG island methylator phenotype (CIMP), which is characterized by simultaneous methylation of the CpG islands of multiple genes, is related to EBV infection. When carcinomas that had high CIMP methylation (CIMP-H) were compared with tumors that had low CIMP methylation (CIMP-L) or negative CIMP methylation (CIMP-N), EBV-associated tumors were found to be associated strongly with CIMP-H, with hypermethylation of tumor-related genes, but there was an inverse relationship with p53 and K-ras mutations, with none of the latter in one study being detected among CIMP-H tumors. Carcinomas that have wild-type K-ras can therefore be expected to be enriched with EBV-associated carcinomas. CIMP-H tumor was also associated with proximal location, diffuse type, and less advanced pathologic TNM status. Patients who had CIMP-N gastric tumors had a significantly worse survival than patients who had CIMP-H tumors or CIMP-L tumors. In addition, among EBV-negative gastric carcinoma subgroups, CIMP-H gastric carcinoma showed comparatively higher frequency of methylation than CIMP-I or CIMP-N, especially of p16 and hMLH1. CIMP-N gastric carcinoma predominantly consisted of advanced carcinoma with significantly higher frequency of lymph node metastasis.68
Finally, one study found that mixed-type carcinomas, which have both diffuse and intestinal components, had more methylated genes than either “pure” diffuse or intestinal carcinomas, and were present in both components of these mixed tumors. Further, this trend was also observed when EBV-positive or microsatellite instability (MSI)-positive carcinomas were excluded from the analysis. and was significantly higher than was found in either intestinal or diffuse carcinomas. These findings suggest that the mixed intestinal-diffuse type of carcinoma may be a distinct subgroup characterized by enhanced CpG island hypermethylation of promoters.69
JC virus. JC virus (JCV) is a polyomavirus that infects humans worldwide, with more than 80% of the adult population having antibodies against it. JCV sequences are frequently present throughout the normal human GI tract and in colorectal and gastric cancers. JCV expresses the T-Ag protein, which in one study was found in half of 90 gastric cancer tissues, but was independent of EBV expression. However, T-Ag expression was detected in a significantly lower percentage of MSI-H cancers (14%) than in non-MSI-H cancers. T-Ag-positive gastric cancers showed a significant increase in the allelic losses and aberrant methylation compared with T-Ag-negative gastric cancers.70
Dietary factors. The dietary factors implicated are high salt consumption, associated with salted fish and meat, smoked foods, and low caloric intake. Smoking and alcohol consumption may also contribute. Nitrosamines, resulting from the conversion of dietary nitrates in the acid milieu of the stomach, have also been proposed but are unconfirmed as important carcinogenic agents in gastric carcinoma.71 Consequently, there has been an interest in the possible role of antioxidants (which can prevent the conversion of nitrates to nitrosamine), such as vitamins C and E and selenium, in lowering the risk of gastric cancer.72
Smoking. Smoking is significantly associated with gastric cancer, especially noncardia cancer, in a dose-dependent manner.73, 74, 75 Moist snuff or smokeless tobacco, which is commonly used in Scandinavia as an alternative to cigarettes, has been shown to enhance noncardia gastric cancer.76
Genetic factors. Individual risk from carcinogen exposure varies as a function of both environmental and inherited genetic factors. Inherited genetic susceptibility is involved in gastric carcinogenesis in two ways: (a) germline mutations involved in hereditary gastric cancer predisposing to either syndromes or other genetic abnormalities that predisposing to gastric cancer and (b) polymorphism in genes.
Hereditary gastric cancer predisposition syndromes. These contribute a small population of gastric cancers and include hereditary nonpolyposis colon cancer syndrome (HNPCC, GAPPS syndrome, Lynch’s syndrome), hereditary diffuse gastric cancer (HDGC), Li-Fraumeni syndrome, familial adenomatous polyposis, Peutz-Jeghers syndrome, and juvenile polyposis (with or without hereditary hemorrhagic telangiectasia (HHT) syndrome), and there are doubtless others with a clear familial trait waiting to be discovered.
HNPCC results from germline mutations in one of the mismatch repair genes (e.g., MLH1, MSH2, MSH6, PMS1, and PMS2) and tumors that arise in association with HNPCC are characterized by MSI. Gastric cancers are said to arise in 11% of HNPCC families and the majority of these cancers are of intestinal type.77
HDGC is the currently known autosomal dominantly inherited disorder characterized by early onset of diffuse gastric cancer (DGC) of signet-ring cell type.78, 79 In prophylactic gastrectomy specimens performed in at-risk family members, “in-situ signetring cell carcinoma” (putative precursor lesions for HDGC), sometimes with pagetoid spread along the gastric glands or foveolae, is commonly identified.79 Female mutation carriers have a risk also for lobular breast cancer.71 HDGC must fulfill one of the following criteria:
1. Two or more cases of documented DGCs in first- or second- degree relatives under the age of 50, or
2. Three or more cases of documented DGC in first- or second-degree relatives independent of the age of diagnosis.80
A germline mutation in the E-cadherin (CDH1) gene has been identified in 30% to 40% of HDGC families.80 CDH1 germline mutation carriers in HDGS have a >70% lifetime risk of developing diffuse-type gastric cancers.81 The inactivation of CDH1 is caused by heterozygous germline mutations of CDH1 and the somatic inactivation of the second CDH1 allele by mechanisms that include DNA promoter hypermethylation.82, 83 The CDH1 gene encodes for E-cadherin, which is a transmembrane protein expressed at the baso-lateral cell surface of the epithelial cells. E-cadherin plays an important role in cell-to-cell adhesion through its extracellular domain and also in tumor suppressor function through the interaction of its intracellular cytoplasmic domain with the catenins. The loss of cell-to-cell adhesion and promotion of tumor invasiveness may follow the loss of E-cadherin.84
GAPPS syndrome (gastric adenocarcinoma and proximal polyposis of the stomach) has proximal fundic gland polyposis but there is nothing else to suggest FAP in this autosomal dominant syndrome, although it seems likely to be the result of activation of the wnt signalling pathway.
Over 50% of families with Li-Fraumeni syndrome have an identifiable germline TP53 mutation85, 85a and patients with Li-Fraumeni syndrome are at increased risk for developing multiple primary cancers. Classic Li-Fraumeni syndrome is defined by the following criteria:
1. A proband with a sarcoma diagnosed before 45 years of age.
2. A first-degree relative with any cancer under 45 years of age.
3. A first- or second-degree relative with any cancer under 45 years of age or a sarcoma at any age.
Familial adenomatous polyposis (FAP) results from germline mutations in the FAP (adenomatous polyposis coli [APC]) tumor suppressor gene on chromosome 5q21-q22. In stomachs of FAP patients, fundic gland polyps are the most common gastric lesions and adenomas are less common. FAP families occasionally develop gastric cancers, particularly in Japanese families. \\86, 87 However, while occasional cases do develop either from antral adenomas or from dysplastic fundic gland polyps in the West,88 they are vanishingly rare so that no significant increased risk is found for gastric cancer in FAP patients in Western countries.87, 89
LGD in fundic gland polyps is quite frequent, but HGD and carcinomas arising in fundic gland polyps seem extremely rare. Interestingly, inflammation and H. pylori are rare in fundic gland polyps whether sporadic or syndromic.
LGD in fundic gland polyps is quite frequent, but HGD and carcinomas arising in fundic gland polyps seem extremely rare. Interestingly, inflammation and H. pylori are rare in fundic gland polyps whether sporadic or syndromic.
Peutz-Jeghers syndrome is an autosomal-dominant disorder characterized by mucocutaneous pigmentation, hamartomatous polyps, and an increased risk of associated malignancies; germline mutations of the tumor suppressor gene LKB1/STK11 are responsible for this syndrome. A meta-analysis of Peutz-Jeghers syndrome showed that cancers having a statistically significant increased RR compared with the general population include esophagus (57), stomach (213), small intestine (520 because small bowel cancer is rare), colon (84), pancreas (132), lung (17), breast (15), endometrium (16), and ovary (27), but not testicular or cervical malignancies.90 These markedly decrease longevity compared to the general population—see Chapter 20.
Juvenile polyposis is usually associated with large bowel polyps, but those with SMAD4 germline abnormalities are associated with gastric juvenile polyps and an increased risk of gastric carcinoma, which may develop as early as the fourth decade of life. Forests of polyps may be present (see subsequent section in this chapter). Carcinoma develops in dysplastic juvenile polyp. Germline testing becomes important for follow-up as surveillance endoscopy is required with this group of patients. It should also be remembered that a combined syndrome of JPS and HHT (termed JPS/HHT) may be present in 15% to 22% of individuals with an SMAD4 mutation, so that the possibility of juvenile polyps and their sequelae need to be considered in patients with HHT and a germline SMAD4.1
Other genetic abnormalities predisposing to gastric cancer. These include DNA aneuploidy, decreased expression of tumor suppressor or protective genes due to promoter hypermethylation (p16, p27, TFF1, and MLH1), amplification of oncogenes, or over-expression of growth factors (Cox-2, HGF, VEGF, EGFR/EGFR, c-Met, and amplified breast cancer-1 or AIB-1).91
Polymorphism in genes. Molecular cancer epidemiology has revealed that genetic polymorphisms influence both response and susceptibility to carcinogens and that these gene-environmental interactions could explain the high variation in gastric cancer incidence around the world.92, 93 Genetic polymorphisms found to be associated with gastric cancer risk include genes in (a) mucosal protection (mucin gene), (b) inflammatory responses (proinflammatory cytokine genes including IL-1β, IL-10, and TNF-α, TLR4, and HLA), (c) carcinogen metabolisms, (d) oxidative damage, and (e) DNA repair.92 Among these, a meta-analysis showed the most consistent results associated with increased gastric cancer risk were IL-1β and NATI (involved in the metabolism of aromatic and heterocyclic amine carcinogens) variants and that polymorphisms in HLA-DQ, TNF-α, CYP2E (involved in the metabolism of low-molecular-weight toxins and N-nitrosamines) might confer some protective effect against gastric cancer.92
Predisposing Conditions
These are pathologic processes associated with an increased risk of cancer. These include atrophic gastritis, subtotal gastrectomy, immunodeficiency syndromes, chronic gastric ulceration, and Menetriér’s disease. It should be stressed that the association of these disorders is controversial and that some conditions, such as atrophic gastritis and postgastrectomy states, may be more relevant to high-risk countries, and that Menetriér’s disease is very poorly defined and likely involves several different entities.
The association of chronic gastric ulceration with carcinoma is controversial and often difficult to prove. In order to demonstrate this association convincingly, it is necessary to demonstrate the typical features of a chronic gastric ulcer with a carcinoma arising from it, usually at one margin. This may be difficult in advanced cases, in which the tumor may have overrun much of the ulcer.
Early studies from Japan frequently reported cancers around ulcers, but now these cancers are rarely seen.47, 94 It is also noteworthy to remember that early gastric cancer may closely resemble a benign peptic ulcer, and heal temporarily with proton pump inhibitors (PPIs).
H. pylori-related chronic atrophic gastritis. This is by far the most important precursor of gastric cancer. Long-term follow-up studies in Finland on patients with atrophic gastritis, with or without pernicious anemia, have shown a 9% incidence of gastric cancer.95 Furthermore, studies in Japan have shown that 80% of gastric cancers arise in atrophic gastric mucosa,96 specifically in areas of incomplete intestinal metaplasia characterized by combined foveolar mucin cells and intestinal goblet cells, the latter having poorly developed microvilli, secretion of sulfomucins, and a lack of Paneth cells.47, 97 This increased risk of gastric carcinoma in patients with severe atrophic gastritis does not appear to be borne out in countries that have lower prevalence rates in general for gastric cancer. The key factor seems to be multifocal intestinal metaplasia on a background of chronic atrophic gastritis. The whole concept of an advancing front of atrophy in chronic H. pylori gastritis, previously thought to be just an aging change, is discussed further in Chapter 13.
A prospective study of 1,526 Japanese participants showed that patients with H. pylori infection and severe atrophic gastritis, corpus-predominant gastritis, or both, along with intestinal metaplasia were at high risk for intestinal-type gastric cancer, and that many of the patients with diffuse-type gastric cancer had atrophic changes and pangastritis.52 Cancers tend to be located in the gastric antrum and lesser curve.
A possible association of diffuse-type gastric cancer in young adults with nodular gastritis has also been suggested from observations in Japanese.98, 99, 100, 101 Nodular gastritis is characterized by the presence of H. pylori infection and follicular gastritis and shows a predilection for females and young adults.98, 99, 101
Postgastrectomy. The risk of carcinoma developing in the gastric remnant following subtotal gastrectomy (almost always in peptic ulcer disease) has been estimated at 5% to 10%. It is said to occur much more frequently following partial gastric resection with Billroth II anastomosis47, 102 and has been blamed on reflux of bile that also contains activated pancreatic juice and ensures reflux of small intestinal bacteria into a stomach secreting little acid, as the G-cell-rich antral area has been removed with consequent atrophy of oxyntic mucosa. It appears to be a late complication, occurring 15 to 25 years after gastrectomy.47, 102 The tumors may occur anywhere in the residual stump or at the stoma and are invariably adenocarcinoma histologically, although there is one report of a squamous cell carcinoma.103 These findings have been disputed by some,104 who in their long-term follow-up studies in the United States have found that the incidence of gastric cancer is no more common among patients with prior gastric surgery for ulcer disease than in the general population. Nevertheless, inflammatory polyps with marked reactive change are common at the anastomotic line, and this tends to be where carcinomas occur. It should also be recalled that as the gastrectomy was likely carried out for “peptic ulcer disease”— a somewhat archaic term that now embraces both H. pylori and medication (NSAIDs, ASA, etc.), many of these patients may well still be harboring H. pylori in their proximal stomach, although the role that this plays in the carcinomas, which are usually much more distal, is unclear and may be minimal.
Immunodeficiency disorders. The risk of cancer in the primary immunodeficiency disorders ranges from 2% to 10%.45 Many of the tumors are lymphomas, but gastric carcinoma also occurs (see Chapter 3). Because the association is usually recognized and patients are clinically followed up, predisposing factors such as H. pylori are invariably eradicated, but carcinomas may still arise in the noninflamed mucosa.
Menetriér’s disease. (See also Chapter 13). There are a number of case reports describing the association of Menetriér’s disease with gastric cancer,105 and we have seen several apparent examples of this (Fig. 14-11). The problem with Menetriér’s disease is that it is a poorly defined disorder in the literature, often merely referring clinically to giant gastric folds. Thus more accurate information about the specific lesions is needed before this condition can be labeled a premalignant condition. In classic forms it is limited to the oxyntic mucosa, and is diffuse without areas of intervening normal mucosa,
thus excluding multiple hyperplastic-type polyps from this diagnosis. However, at least some patients with cancer and a diagnosis of Menetrier’s disease are very likely examples of juvenile polyposis with predominant or isolated gastric involvement. As with most gastric diseases there are two questions: (a) Is Helicobacter present, and if it is then eradicate it, and see if the underlying disease improves. If not (b) is there a lymphocytic gastritis that may indicate undetected H. pylori requiring serology. If so again it should be eradicated. Prolonged antisecretory therapy may be required after eradication. If there is no H. pylori infection or inflammation and serology is negative, then PPIs are often given “on spec.” This condition is discussed further in Chapter 13. More recently, TGF-a or epidermal growth factor was shown to be significantly up-regulated in Menetriér’s disease.106 This resulted in selective expansion of surface mucus cells in the fundus and the body of stomach. These patients have been successfully treated with a blocking monoclonal antibody specific to EGFR (C225).
thus excluding multiple hyperplastic-type polyps from this diagnosis. However, at least some patients with cancer and a diagnosis of Menetrier’s disease are very likely examples of juvenile polyposis with predominant or isolated gastric involvement. As with most gastric diseases there are two questions: (a) Is Helicobacter present, and if it is then eradicate it, and see if the underlying disease improves. If not (b) is there a lymphocytic gastritis that may indicate undetected H. pylori requiring serology. If so again it should be eradicated. Prolonged antisecretory therapy may be required after eradication. If there is no H. pylori infection or inflammation and serology is negative, then PPIs are often given “on spec.” This condition is discussed further in Chapter 13. More recently, TGF-a or epidermal growth factor was shown to be significantly up-regulated in Menetriér’s disease.106 This resulted in selective expansion of surface mucus cells in the fundus and the body of stomach. These patients have been successfully treated with a blocking monoclonal antibody specific to EGFR (C225).
Premalignant Lesions of the Stomach
Premalignant lesions have morphologic features of neoplasia and have the potential to become cancerous. They usually arise in individuals who have one or two predisposing conditions and probably represent the final common pathway through which these conditions give rise to cancer.
Adenoma/dysplasia/intraepithelial neoplasm. Gastric adenomas are considered to carry an unusually high risk of carcinoma. The larger the polyp, and higher the grade of dysplasia, the higher is the frequency of malignant transformation. Overall about 35% of gastric adenomatous polyps show intramucosal carcinoma/high grade dysplasia, in contrast to 4% to 5% of hyperplastic polyps.107 This is discussed in detail in the previous section on adenomas.
Gastric polyps. Gastric polyps other than adenomas, such as fundic gland polyps, especially in patients with FAP and those of familial juvenile polyposis, may also on occasion show dysplasia and malignant transformation, as may a small proportion of sporadic hyperplastic polyps (see subsequent discussion).
Gross Features of Gastric Carcinoma
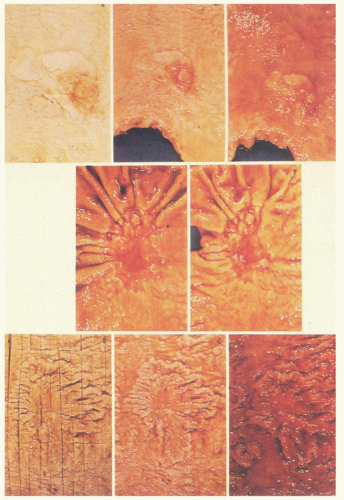
Macroscopically, a number of classifications have been proposed for gastric carcinoma, but the most widely adopted or modified is that of Borrmann (Fig. 14-12).108 Although a fifth category is sometimes added for those that do not fit (unclassifiable). Type 0 was also added for superficial tumors (Fig. 14-12)109, 110:
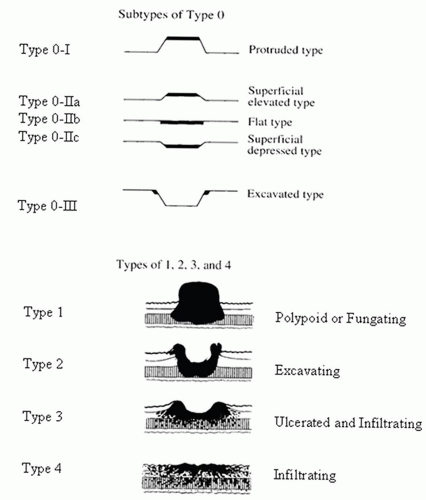 Figure 14-12. Macroscopic types of gastric cancer. The original Borrmann classification are the types 1 to 4, while the subtypes of early gastric carcinoma (superficial flat carcinomas, type 0) were added subsequently and reflect the same principles. (Reprinted from Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 2nd English edition. Gastric Cancer 1998;10-24, p. 12 Figs. 3 and 4, with permission.) |
Type 0—Superficial, flat tumors with or without minimal elevation or depression
Type 1—Polypoid tumors
Type 2—Ulcerated carcinoma with sharply determined and raised margins
Type 3—Ulcerated carcinoma without definite limits, infiltrating into the wall
Type 4—Diffusely infiltrating carcinomas in which ulceration is usually not marked
Type 5—Unclassifiable carcinomas that do not fit into any of the above types
Types 1, 2, 3, and 4 correspond to the four classes found in Borrmann’s classification for advanced gastric cancer.108
Advanced gastric carcinomas are characterized by exophytic intraluminal or infiltrative growth patterns modified by surface erosion and ulceration.47, 94 Some advanced carcinomas reveal macroscopically superficial flat tumors resembling early gastric cancers and they are also described as subtypes of type 0 (Fig. 14-13).
Gross Features of Superficial Cancers and Their Subdivision
These tumors are sometimes referred to as “type 0 gastric cancers,” especially in Japan, and can also be divided into the usual three main groups:
Protruded type (type 0-I)
Superficial type (type 0-II) with yet three further subdivisions, 0-IIa, 0-IIb, and 0-IIc
Excavated type (type 0-III) (Fig. 14-13)110
The superficial type of tumor is by far the most common, accounting for just under 80% of early gastric cancer; the other two types account for about 10% each.47, 111
The subdivisions of type 0-II are
Type 0-IIa—superficial elevated that has a mucosa approximately up to twice as thick as the surrounding mucosa (practically 2-3 mm in thickness)
Type 0-IIb—flat
Type 0-IIc—slightly depressed due to surface erosion or mucosal atrophy (Fig. 14-13)
The excavated type 0-III is characterized by a deep ulcer with a rim of narrow margin of cancer. In the combined superficial types, the type occupying the largest area is described first, followed by the next type, for example, IIc+III.
Although there is an overall correlation between these subtypes and long-term prognosis, with type 0-I having the best prognosis and type 0-III the worst, the ultimate prognosis is related to the depth of invasion of the tumor (see next section).With the exception of the ulcerating and diffusely infiltrating tumors, which do appear to behave in a more aggressive manner, there is little evidence that the gross morphology of gastric adenocarcinomas is of any prognostic significance. Thus a too precise macroscopic classification of gross lesions is of limited practical value, especially since there is often a mixture of gross types in any one case.
Classification and Natural History of Early and Late (Advanced) Gastric Carcinoma
Early gastric carcinoma/cancer. Early gastric cancer has been defined as a gastric neoplasm confined to the mucosa and submucosa, irrespective of whether lymph node metastases are present. Histologically, both early and advanced gastric cancer shows the same histologic spectrum. Tsukuma et al.112 followed 43 cases of early gastric cancers from 6 to 88 months in patients refusing resection. They found that 16 (37%) remained unchanged, while the remaining 27 developed into advanced carcinomas. These results and those of similar studies by others suggest that the two types of cancer are the same entity but at different stages of development.
Early gastric carcinoma can be subdivided histologically into intramucosal carcinoma, in which there is invasion into the lamina propria or muscularis mucosae, and submucosal invasion. Most of the tumors occur in the antrum and lesser curvature, and about 10% are multifocal. In Japan, approximately 50% of all gastric cancers fall into this category.113, 114 In Western series, the incidence of early gastric cancer is reported to be around 10% to 20% of all gastric carcinomas.115
There is a rough correlation between the subtypes of early gastric cancer and advanced cancers. Polypoid early lesions tend to give rise to advanced polypoid carcinomas, and type II lesions, with the exception of the large eroded type, give rise to ulcerative infiltrating carcinomas. The large eroded type and the excavated types may result in a wider variety of advanced cancers, ranging from the ulcerative infiltrating to the diffusely infiltrative carcinomas.47
There is also a fairly close correlation between macroscopic appearance, histologic type, and depth of invasion.47, 116 Polypoid or elevated lesions are usually well differentiated, whereas excavated and large, depressed lesions are often poorly differentiated. Similarly, small lesions (<3 cm) with demarcated margins are usually well differentiated and show no or little invasion, whereas larger lesions with serrated margins are often poorly differentiated, with signetring cells, and invade into the submucosa.47, 117
Advanced gastric cancer. Advanced gastric cancer is defined as a gastric neoplasm invading the gastric wall beyond the submucosa (into the muscularis propria or beyond)
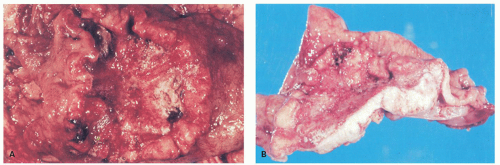
At the time of presentation, advanced gastric cancer is usually readily visible endoscopically, forming obvious lesions that still form the bulk of resections (Fig. 14-14), and is often associated with vague symptoms such as weight loss and anemia. Other modes of presentation consist of ulcer-type pain, occult or overt GI bleeding, or gastric outlet obstruction if the tumors are near the pylorus. Some patients present with widely metastatic disease.
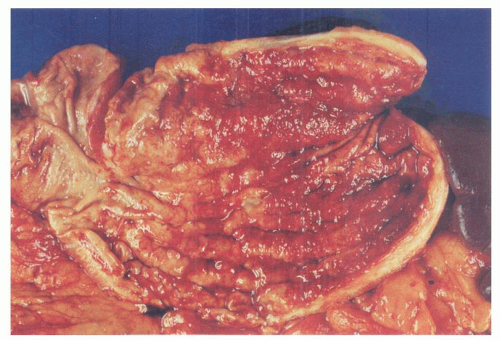
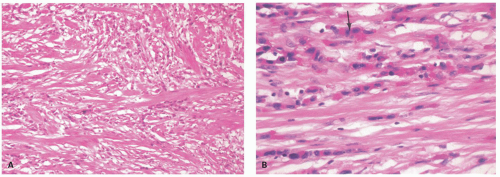
Linitis plastica (leather bottle stomach) is a diffusely infiltrative carcinoma, usually involving the greater part of the stomach, associated with a marked desmoplastic reaction and resulting in a grossly thickened stomach, which has been likened to a leather bottle (Fig. 14-15). Frequently there is also a concomitant giant hypertrophy of the mucosal folds. Because of the intense fibrosis, the often undifferentiated nature of the tumor cells, and the associated mucosal ulceration, these tumors can be misdiagnosed as chronic peptic ulcer. The exact origin of these tumors within the stomach can be impossible to find.
As with early gastric cancers, there is some correlation between gross characteristics, histologic appearances, and the age of the patient. The exophytic and expanding lesions tend to be well or moderately well-differentiated, associated with intestinal metaplasia, and found in elderly patients, whereas the diffusely thickened and infiltrative lesions are often poorly differentiated and tend to occur in younger patients. Superficial spreading carcinomas make up about 3% of gastric cancers. As stated previously, they can measure up to 10 cm in diameter, but are confined to the mucosa and submucosa and behave like early gastric cancers.
The size of advanced gastric cancers at the time of diagnosis can vary from <2 to more than 15 cm in diameter and can affect the entire stomach (linitis plastica). The majority are in the 2- to 6-cm (44%) and 6- to 10-cm range (30%).118
Histologic Classification of Gastric Carcinomas
Lauren classification. Because of the limitations of the morphologic classifications in regard to reproducibility and clinical outcome, a number of simplified classifications have been proposed. Lauren119 proposed classifying advanced gastric carcinomas into two types: intestinal and diffuse.
The intestinal type (presumed to be derived from intestinalized gastric mucosa) is more common in males and older age groups. It usually has a polypoid or fungating gross appearance and often an expansile growth pattern. Histologically, it is characterized by well-defined glandular structures and is almost invariably associated with intestinal metaplasia and variable degree of atrophic gastritis (Fig. 14-16). It shows preferential metastasis to the liver.
In contrast, the diffuse type (thought to arise from native gastric cells) shows a more equal male-to-female ratio and is more frequent in younger individuals. It has an ulcerative or infiltrative gross appearance and a diffuse, infiltrative growth pattern. Histologically, it is usually a poorly differentiated adenocarcinoma, often with signet-ring cells (see subsequent discussion) and features of linitis plastic (Fig. 14-17). It tends to metastasize to the peritoneal cavity. While Lauren’s
classification has not revealed differences in survival, it has proven useful for comparative epidemiologic studies, although about 15% of cases show an overlap of patterns and are impossible to classify.119
classification has not revealed differences in survival, it has proven useful for comparative epidemiologic studies, although about 15% of cases show an overlap of patterns and are impossible to classify.119
Nakamura classification. Nakamura et al. classified gastric carcinoma into two types based on the histogenesis and biologic characteristics: differentiated and undifferentiated carcinoma.120, 121 They correspond to Lauren’s intestinal and diffuse tumor types, respectively.
Ming classification. Ming122 divided advanced gastric cancer into expansile and infiltrative types according to their microscopic growth patterns (Figs. 14-18 and 14-19). In general, Ming’s expansile and infiltrative tumors correspond to Lauren’s intestinal and diffuse tumor types, with a similar number of cases showing overlap in patterns.
WHO histologic classification and grading of gastric cancer. The World Health Organization (WHO) divides gastric carcinoma into four main histologic types of adenocarcinoma (papillary, tubular, mucinous, and signet-ring carcinoma) (Fig. 14-20) and rare variants (see below).1 The diagnosis is based on the predominant histologic type. It is still uncertain whether, stage for stage, the different types of gastric adenocarcinoma affect the prognosis. This may in part be due to variation in the histologic type of gastric cancer from one area to another. For example, an adenocarcinoma may have a papillary pattern superficially and may be mucinous in the deeper portions.
HISTOLOGICAL SUBTYPES OF CARCINOMA
Tubular Adenocarcinoma
This type is composed predominantly of branching tubules resembling colonic epithelium and, less commonly, small glandular structures resembling antral glands (Fig. 14-20A). The glands may be cystically dilated with mucus. The tumor cells are columnar, cuboidal, or flattened and contain variable amounts of intracytoplasmic mucin.
The WHO classification of tumor differentiation is based on the grades of glandular differentiation. Thus this grading system also applies primarily to tubular adenocarcinoma as follows.
Well-differentiated Adenocarcinoma
These tumors show well-developed glands lined by tall columnar cells. Ultrastructurally, they have well-defined cell junctions. They are rich in capillaries and sparse in stroma, and basically resemble colorectal tumors (Fig. 14-20A).
Moderately Well-differentiated Adenocarcinoma
These tumors are intermediate in appearance between well- and poorly differentiated adenocarcinomas. The glandular origin of these tumors is obvious, but the glands are less well formed with cribriform or acinar forms. The amount of stroma is variable.
Poorly Differentiated Adenocarcinoma
In these tumors, gland formation is poor and the dominant pattern is one of anastomosing trabeculae, small or large solid clusters, or single cells (Fig. 14-20B,C). Cytologically, the tumor cells are small and immature, and ultrastructurally have scant microvilli
and small mucin granules and lack tight junctions. As a rule, these tumors arise in nonmetaplastic gastric mucosa, and tend to infiltrate the stroma diffusely, eliciting a dense desmoplastic reaction.
and small mucin granules and lack tight junctions. As a rule, these tumors arise in nonmetaplastic gastric mucosa, and tend to infiltrate the stroma diffusely, eliciting a dense desmoplastic reaction.
Papillary Adenocarcinoma
This type is composed of villous-like epithelial processes with fibrovascular cores. The tumor cells are cylindrical or cuboidal, with or without well-formed, striated borders, and frequently secrete mucus as small droplets (intestinal type), or cylindrical or cuboidal mucous cells (gastric type). They may also contain goblet cells. These tumors typically grow as polypoid masses protruding into the gastric lumen, and deeply in an expansile manner, with pushing margins (Fig. 14-20D).
Mucinous Adenocarcinoma (syn. Mucoid, Mucus, Colloid, and Muconodular Adenocarcinoma)
These tumors produce abundant intracellular and extracellular mucus, which may be visible grossly. By definition, mucinous stroma occupies more than 50% of the tumor. They may show two growth patterns: (a) glands lined by mucus-secreting carcinoma cells with extracellular mucus (Fig. 14-20E) and (b) scant fragments of disrupted glands or cell clusters floating in extracellular mucus pool (Fig. 14-20F). Signet-ring cells may be found in some of these tumors, but do not dominate carcinoma tissue.
Signet-ring Cell Carcinoma
In this type of carcinoma, more than 50% of the tumor consists of carcinoma cells with varying degrees of intracytoplasmic mucin that may present in isolated or small groups. They may consist of the following types of cells independently or in combination within any one tumor123: (a) surface mucous cell type (foveolar cell type) that has a small- to medium-sized faintly reticular cytoplasm rich in neutral mucins (surface mucous cell-type mucins), (b) gland mucous cell type that has a small- to medium-sized faintly eosinophilic granular cytoplasm rich in neutral or acid mucins (gland mucous cell-type mucins), (c) goblet cell type that has medium-sized to large clear cytoplasm rich in acid mucin, (d) microcystic type that has intracellular microcyst or intracellular lumina with small amount
of mucins lining the inner surface of microcysts, and (e) immature type that has a small cytoplasm containing only a small amount of mucins and exhibiting a high N/C ratio (Figs. 14-10 and 14-21).
of mucins lining the inner surface of microcysts, and (e) immature type that has a small cytoplasm containing only a small amount of mucins and exhibiting a high N/C ratio (Figs. 14-10 and 14-21).
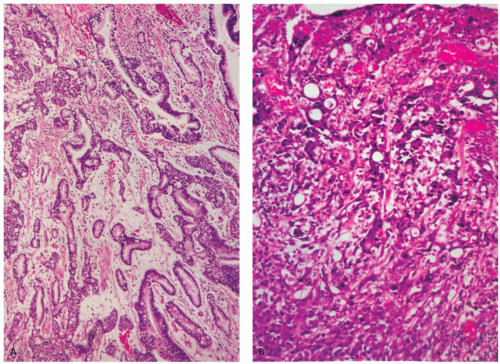 Figure 14-20. Histologic spectrum of gastric carcinoma. A: Well-differentiated tubular adenocarcinoma. B: Poorly differentiated adenocarcinoma containing anastomosing tubules of tumor cells. |
The detection of these malignant cells is sometimes subtle and they are easily missed, as they may occur in otherwise normal mucosa. They can be enhanced using mucin stains, ideally PAS stain, with or without AB at pH 2.5, the latter staining acid mucins (Fig. 14-21B,J). PAS stains both neutral and acid mucins found in normal superficial and deep glands in the stomach. Many signet-ring carcinomas would be expected to contain only neutral mucins, and this is sometimes the case. However, they may also contain acid mucins typically found in goblet cells; so they sometimes also stain with mucicarmine or AB (AB pH 2.5) stains or a combined AB-PAS stain. However, sometimes they contain only acid mucin, as is typically seen in normal gastric mucosa; so they do not stain with either AB or mucicarmine. If mucicarmine is used as “the” mucin stain, it will not stain tumor cells only producing neutral mucins, leading to a false-negative mucin stain, and so is best avoided,) unless used with a PAS stain. Immunostaining for low molecular weight or pancytokeratin is also helpful in highlighting tumor cells in the lamina propria, and is preferred as some diffuse and signetring carcinomas can lose most or all of their mucin in the deeper part of the mucosa. In fact, immunostains have largely replaced traditional mucin stains when available due to their higher sensitivity and specificity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree