Gastric Carcinoid
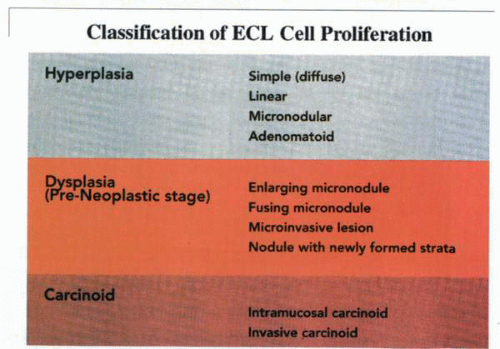
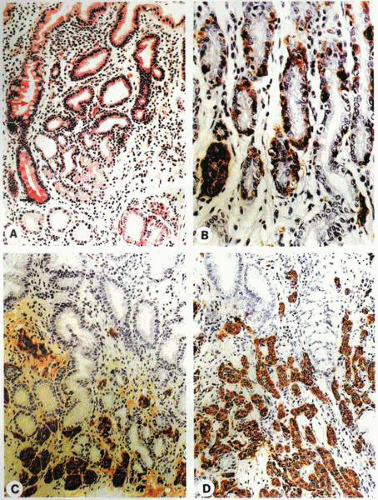
It is apparent that during proliferation of the ECL cells, a definable morphologic pattern evolves in the course of the transformation from the normal cell to the neoplastic mode. In 1988, Solcia and others delineated a system of classification for the spectrum of proliferative changes of endocrine cells of the gastric fundus. This classification is defined by histopathologic appearances ranging from hyperplasia to dysplasia to neoplasia.
Histology
The term pseudohyperplasia has been used to describe cell clustering unassociated with cell proliferation. The successive stages of hyperplasia are termed simple, linear or chain-forming, micronodular, and adenomatoid. Dysplasia is characterized by relatively atypical cells with features of enlarging or fusing micronodules, microinvasion, or newly formed stroma. When the nodules increase further in size to larger than 5 mm or invade into the submucosa, the lesion is classified as a neoplasm. This stage is divided into intramucosal and invasive forms.
The entire spectrum of endocrine cell proliferation, from hyperplasia to dysplasia and neoplasia, has been observed in MEN-1-ZES and diffuse type A chronic atrophic gastritis. Both hyperplastic and pseudohyperplastic changes occur with low frequency in the H. pylori-related chronic gastritis associated with ulcer disease or dyspepsia.
However, because no progression to dysplastic or neoplastic lesions has thus far been documented in these latter conditions, their role in gastric endocrine tumorigenesis appears unlikely. Carcinoid tumor is thus characterized morphologically by the criteria of size and invasion.
Biologically, however, the state of neoplasia is recognized by autonomy from the trophic effects of gastrin. A lesion that fails to regress once gastrin levels have been normalized is therefore regarded as a carcinoid tumor.
Development
In rats and Mastomys, it appears that a threshold value of plasma gastrin is necessary not only for ECL cell hyperplasia to occur but also for conversion to gastric carcinoid. This value reflects not only gastrin levels but also length of exposure and other factors in which female gender and, in humans and Mastomys, genomic events (MEN-1) are involved. In fact, in humans, the ECL cell density does not exhibit a relation to plasma gastrin levels in normogastrinemic individuals, nor does it reveal any significant differences in duodenal ulcer patients with mild hypergastrinemia. In patients on PPIs for 6 months, no significant changes in ECL cells were noted.
Furthermore, in peptic ulcer patients receiving omeprazole for more than a decade, ECL cells were not significantly affected. If plasma gastrin levels of greater than 400 pg per mL are present, significant proliferation not progressing to dysplasia has been noted. It has therefore been suggested that in patients with plasma gastrin levels greater than 400 pg per mL, gastric biopsy should be undertaken to evaluate endocrine cell status, or a decrease in the dosage of the PPIs should be considered. In patients with sustained hypergastrinemia associated with surgical vagotomy, alterations in ECL status are not significant. Although duration of exposure appears important, it has been noted that ECL cell hyperplasia may be stable with time, provided that gastrin levels do not increase significantly. With hypergastrinemia alone (e.g., sporadic ZES), ECL cell proliferation is usually limited to hyperplastic lesions of the simple or linear type. However, in patients with MEN-1-ZES or type A chronic atrophic gastritis, ECL cell lesions are usually dysplastic or overtly carcinoid in nature. Factors other than hypergastrinemia must therefore be involved in ECL cell transformation. In the MEN-1 syndrome, the loss of the tumor suppressor oncogene (menin) on chromosome 11q13 may be related.
Similarly, in atrophic gastritis, either achlorhydria or alterations in the biology of the associated mucosa, which have also been suggested to be implicated in the pathogenesis of gastric adenocarcinoma, may be involved. It has been suggested that detection of dysplastic changes or ECL cell carcinoids in a ZES patient warrant screening of both the patient and the family for a covert MEN-1 syndrome.
The hyperplastic effect of hypergastrinemia on gastric mucosal epithelial cells appears not to be restricted to the ECL cells. Foveolar cells and surface mucous cells also undergo hyperplasia in the human stomach under the influence of excessive gastrin drive, often to a considerable degree. This process may be patchy and not infrequently results in the development of endoscopically detectable polyps that are classified histologically as foveolar hyperplasia. Thus, to add to the confusion surrounding gastric carcinoid tumors, in patients with these lesions arising in a setting of hypergastrinemia, at least two types of mucosal polyps can be expected: those due to ECL cell proliferations and simple foveolar hyperplasias. Whether these foveolar cell hyperplasias represent an early stage on the pathway to gastric adenocarcinoma of the type that occurs with increased frequency in patients with chronic atrophic gastritis type A and pernicious anemia is not known.
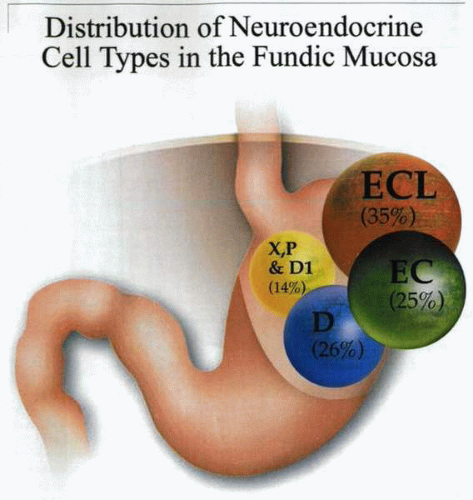
In a morphometric ultrastructural investigation of sporadic ZES patients, a 168% increase in the volume density of the total oxyntic endocrine cell population
was noted. This increase was exclusively due to ECL cells, with other types showing a relatively decreased volume. However, in patients with H. pylori, the P and D1 cells have been reported to exhibit hyperplasia. This may reflect the metaplastic background in which endocrine cells proliferate.
was noted. This increase was exclusively due to ECL cells, with other types showing a relatively decreased volume. However, in patients with H. pylori, the P and D1 cells have been reported to exhibit hyperplasia. This may reflect the metaplastic background in which endocrine cells proliferate.
The oncoprotein, BCL2, enhances cell survival by blocking programmed cell death (apoptosis) due to inhibition of mitochondrial export of cytochrome c. Immunohistochemical expression of BCL2 in endocrine cells of the human oxyntic mucosa has been investigated in patients with normal gastric mucosa, sporadic ZES, MEN-1-ZES, hypergastrinemic atrophic gastritis (HAG), and ECL cell carcinoids. In all patients, BCL2 immunoreactivity was exclusively located in endocrine cells located in the middle layer of the oxyntic mucosa.
The ratio of BCL2 to chromogranin A-reacting cells was low in sporadic ZES (no risk of carcinoid). It was, however, maintained in MEN-1-ZES and increased in chronic atrophic gastritis A (conditions with intermediate or high risk of carcinoid, respectively). BCL2 expression by gastric carcinoid markedly varied from one tumor to another, even in the same patient, but was low or absent in most cases. Thus, BCL2 is expressed by normal fundic endocrine cells whose intraglandular location suggests a role for the protein during migration from the neck stem zone to fully differentiated cells at the gland bottom.
Experimental model Mastomys natalensis
A novel rodent model available for study of the ECL cell and particularly its neoplastic transformation is the Mastomys natalensis. This is a sub-Saharan rodent that was initially used in the study of African plague vectors. During these studies, it was noted that a substantial percentage of the animals died of a gastric neoplasm. Initially, this lesion was identified as a gastric adenocarcinoma. It subsequently became apparent that the cell type was more compatible with a carcinoid lesion. Further investigation identified this neoplasm as a histamine-producing ECL cell tumor (ECLoma).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree