Fine Needle Aspiration of a Breast Mass
Judy C. Pang
Claire W. Michael
DEFINITION
Fine needle aspiration (FNA) biopsy is a percutaneous procedure that uses a fine gauge needle with or without a syringe to sample fluid from a cyst or extract cells from a solid palpable mass for cytologic analysis.
PATIENT HISTORY AND PHYSICAL FINDINGS
A focused history should be obtained from the patient including duration of the mass, changes in size, associated pain, or fluctuations of the mass with menstrual cycle. Prior history of trauma or malignancies should also be ascertained. On physical examination, localizing the mass as within the breast parenchyma, lower axilla, or subcutaneous/cutaneous tissue of the chest wall is important. The differential diagnoses may be different. In addition, noting any skin changes such as redness, warmth, or edema is also helpful. Determining the size and quality of the mass as well as depth and relation to other structures is essential for an adequate sample while minimizing complications. There are no absolute contraindications to FNA.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Mammographic and ultrasound findings can be helpful in arriving at an accurate diagnosis. Knowing whether a lesion is solid or cystic can help select the appropriate needle and syringe. For lesions that are nonpalpable or difficult to palpate, image-guided (i.e., ultrasound) FNA is recommended to ensure proper sampling of the mass.
DIFFERENTIAL DIAGNOSIS
Benign (i.e., fibroadenoma, cyst)
Malignant (i.e., carcinoma, lymphoma)
Atypical (core biopsy or surgical excision required for definitive diagnosis)
NONOPERATIVE MANAGEMENT
For patients who opt not to undergo a biopsy, short-term follow-up (4 to 6 months) with repeat imaging and clinical examination to document stability or changes is recommended.
SURGICAL MANAGEMENT
Alternative procedures to FNA biopsy are core needle biopsy and surgical excision of mass.
For solid masses, FNA biopsy provides cells for cytology, whereas core needle biopsy obtains tissue. In situations where an experienced cytopathologist is not available or tissue architecture is necessary to make a diagnosis (e.g., differentiating between in situ and invasive disease), core needle biopsy is preferred.
Surgical excision should be reserved for cases where FNA or core needle biopsy was inconclusive. It may be considered for small breast masses where the patient is strongly desirous of excision.
Preoperative Planning
Prior to the FNA, the location of the palpable mass should be confirmed with the patient. The mass should be examined in the upright and supine position to determine the ideal position for the biopsy.
Positioning
The patient may be upright or supine depending on the location of the mass. The patient should be positioned to optimize palpation and sampling of the mass.
Approach
FNA can be performed using (1) a needle, syringe, and syringe holder; (2) a needle and syringe; or (3) a needle only.
TECHNIQUES
EQUIPMENT
Alcohol pads to cleanse the skin and gauze pads to apply pressure after completion of the procedure
Local anesthetic is optional.
Beveled hypodermic needles
A 23-gauge needle is preferred and typically the one to start with. If inadequate material is obtained, a 22-gauge needle can be used especially for lesions with minimal stroma (i.e., lymphoma, melanoma) or a 25-gauge needle for rubbery or fibrous masses (i.e., fibroadenoma).
The length of the needle is typically 5/8 in to 11/2 in, which is just long enough to reach the target. Shorter needles are easier to manipulate because they will not bend.
A slip-tip syringe is best as it is easy to handle and provides a good seal. A Luer lock syringe may also be used, but it can be difficult to remove the needle. A 10-mL syringe is preferred as it allows the hand to be closer to the target and only 2 to 4 mL of suction is needed for aspiration. For larger cystic lesions, a 20-mL syringe may be advantageous.
A syringe holder allows for one-handed grip and application of suction leaving the other hand free to stabilize the target.
Glass slides and cover slips
Slide holder for air-dried slides
Ninety-five percent ethanol (EtOH) in a jar for fixation of slides or spray fixative. If the jar is not slotted for separating slides, using paper clips on alternating slides can achieve the same goal.
There are different rapid stains that can be used for adequacy checks including toluidine blue, rapid hematoxylin and eosin, rapid Papanicolaou for fixed slides, and Giemsa and Diff-Quik for air-dried slides.
Needle rinses can be performed in RPMI (cell block or flow cytometry for lymphoma), 10% buffered formalin (cell block), or CytoLyt (thin prep).
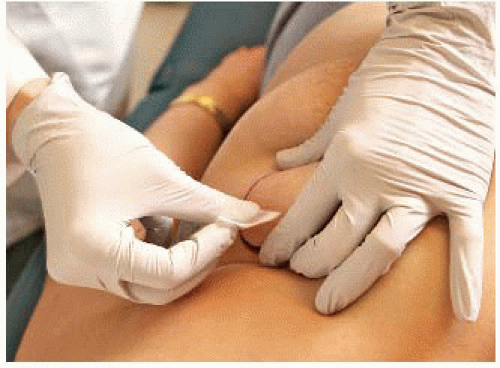
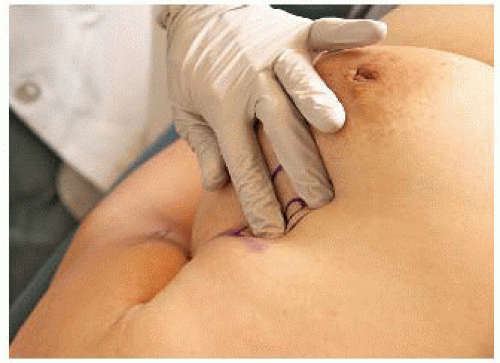
Carefully palpate the mass to estimate the size and depth as well as assess the structures nearby to avoid (i.e., major blood vessels, bone, and lung especially with small breasts).
Fix the mass firmly in place with the fingers.
For large lesions, use the thumb and opposing finger (FIG 1).
For smaller lesions, place the forefinger and middle finger on top of the mass and then spread them apart, stretching the skin (FIG 2).
Plan the angle of the needle at the entrance point of the skin and determine the depth of penetration.
If the needle enters at 90 degrees to the mass, the needle should penetrate the skin on top of the mass (FIG 3A,B).
If the needle enters at a 30- to 45-degree angle, which is oftentimes more comfortable and practical, compensate for the acute angle by penetrating the skin adjacent to the mass and not on top of the mass (FIG 4A,B).
When entering at 90 degrees, penetrating too deep with the needle can potentially result in a pneumothorax. If this is a concern (e.g., mass near the chest wall), a 30- to 45-degree angle is preferred.
To stabilize the instrument, rest the barrel of the syringe on the forefinger of the palpating hand or use the thumb to stabilize the syringe as you enter the mass. Once the needle is in the mass, the thumb can be removed (FIG 4A).
Extracting material
For cystic lesions, applying suction without back and forth movement is sufficient.
For solid masses, 15 to 20 excursions are made before suction is released and the needle is removed from the mass. If blood is seen at the hub, the number
of excursions should be limited and suction released before reaching 15 to 20. Always release the suction before pulling the needle out of the patient; otherwise, all the material will flow into the barrel of the syringe, which will be very difficult to extract (FIG 5).
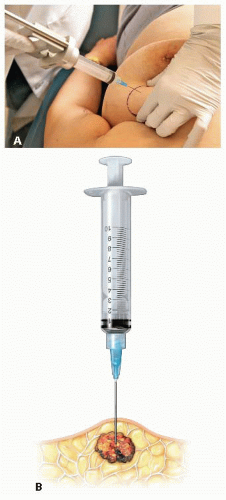
FIG 3 • A,B. Needle entering 90 degrees to the mass should penetrate the skin on top of the mass.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access