Objectives
- Describe oogenesis, its relationship to follicular maturation, and the roles of pituitary and ovarian factors in their regulation.
- Describe gonadotropin control of ovarian function.
- List the target organs and principal physiologic actions of estrogen and progesterone and how they interact with each other.
- Describe the cellular mechanisms of action for estrogen and progesterone.
- Describe the endometrial (proliferative and secretory phases) and ovarian events that occur throughout the menstrual cycle and correlate them with the changes in blood levels of pituitary and ovarian hormones.
- Identify the pathways of sperm and egg transport required for fertilization and for movement of the embryo to the uterus.
- Describe the principal endocrine functions of the placenta, particularly in rescue of the corpus luteum and maintenance of pregnancy, and the fetal adrenal-placental interactions involved in estrogen production.
- Understand the roles of oxytocin, relaxin, and prostaglandins in the initiation and maintenance of parturition.
- Explain the hormonal regulation of mammary gland development during puberty, pregnancy, and lactation, and explain the mechanisms that control milk production and secretion.
- Explain the physiologic basis for the effects of steroid hormone contraceptive methods.
- Describe the age-related changes in the female reproductive system, including the mechanisms responsible for these changes, throughout life from fetal development to senescence.
Female Reproductive System: Introduction
The principal functions of the female reproductive system are to produce the ova for sperm fertilization and to provide the appropriate conditions for embryo implantation, fetal growth and development, and birth. Endocrine regulation of the reproductive system is directed by the hypothalamic-pituitary-ovarian axis. Ovarian-derived hormones regulate the hypothalamic-pituitary-gonadal axis in a classical negative feedback pattern. Throughout the ovarian cycle, a selected follicle is stimulated to undergo growth and development, culminating in ovulation. The remnants of the follicle undergo reorganization into the corpus luteum, a temporary endocrine organ that plays a central role in preparation and maintenance of the initial stages of pregnancy. Parallel changes occur in endometrial morphology and function throughout the ovarian cycle in preparation for implantation of a fertilized ovum. Ovarian and placental hormones maintain pregnancy and prepare the breast for lactation. This chapter describes the basic principles of the neuroendocrine regulation of this hypothalamic-pituitary-ovarian axis.
Functional Anatomy
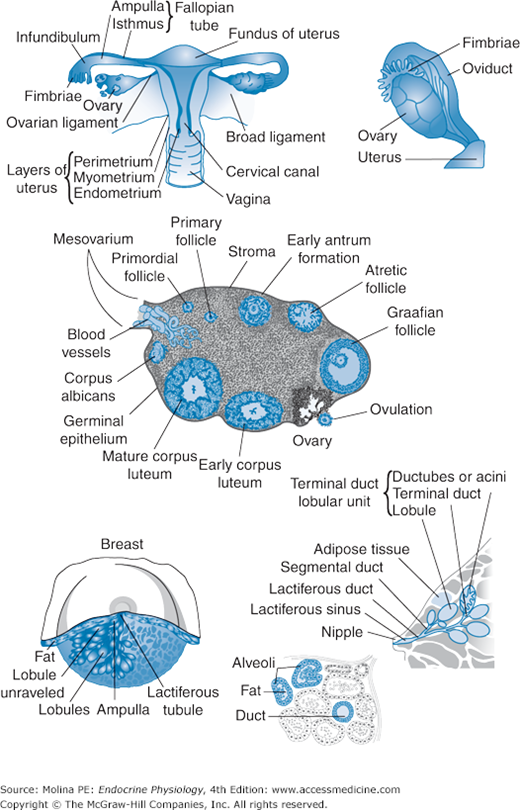
The female reproductive organs include the ovaries, the uterus and fallopian tubes, and the breasts or mammary glands (Figure 9–1). Their growth, development, and function are under hormonal regulation. The ovaries store and release the ovum and produce the 2 principal female sex hormones, estrogen and progesterone. Functionally, the ovaries consist of an outer cortex layer containing different-size follicles and an inner medulla consisting of vascular connective tissue and hilar cells. The primordial follicle contains a primary oocyte surrounded by epithelial (pregranulosa) cells separated from the ovarian stroma by a basement membrane. During follicular development, the epithelial cells differentiate into granulosa cells, and a layer of cells from the ovarian stroma is transformed into theca cells. The larger, more mature follicles are filled with a transparent albuminous fluid and consist of an external fibrovascular coat, connected with the surrounding stroma of the ovary by a network of blood vessels; and an internal coat, consisting of several layers of nucleated cells (granulosa cells) anchored in the zona pellucida, a glycoprotein-rich eosinophilic material surrounding the oocyte. The zona pellucida forms the corona radiata, which close to the time of ovulation is separated from the granulosa cells and expelled with the oocyte. Formation of the follicles begins before birth, and their development and maturation continue uninterrupted from puberty until the end of a woman’s reproductive life, as discussed later.
Figure 9–1.
Functional anatomy of the female reproductive tract. The female reproductive organs include the ovaries, the uterus and fallopian tubes, and the breasts or mammary glands. The ovaries consist of an outer cortex layer that contains different-sized follicles and their remains undergoing apoptosis, embedded in connective tissue. The fallopian tubes extend from each of the superior angles of the uterus and consist of the isthmus, the ampulla, and the infundibulum, which opens into the abdominal cavity and is surrounded by ovarian fimbriae and attached to the ovary. The cilia of the epithelial lining of the fallopian tubes contribute to sperm movement, aiding in fertilization, and facilitate movement of the zygote (fertilized ovum) to the uterus for implantation and fetal development. The breast is organized into lobes made of lobules, connected by connective tissue, blood vessels, and ducts. The lobules consist of a cluster of rounded alveoli, which open into excretory lactiferous ducts and unite to form larger ducts made of longitudinal and transverse elastic fibers. These ducts converge toward the areola, beneath which they form ampullar dilatations, which serve as reservoirs for the milk.
The female genital tract is derived from the müllerian ducts (see Chapter 8; Figure 8–6) and consists of the uterus, fallopian tubes, and vagina (see Figure 9–1). The fallopian tubes extend from each of the superior angles of the uterus and consist of the isthmus, the ampulla, and the infundibulum, which opens into the abdominal cavity and is surrounded by ovarian fimbriae and attached to the ovary (see Figure 9–1). The epithelial lining of the fallopian tubes has secretory and ciliated cells that contribute to sperm movement, aiding in fertilization and facilitating the movement of the zygote (fertilized ovum) to the uterus for implantation and fetal development. These functions are also aided by the rhythmic contraction of the smooth muscle walls.
The uterus is composed of 3 layers: serous, muscular, and mucous. The muscular coat accounts for the bulk of the uterus and consists of bundles of smooth muscle fibers, organized in layers and intermixed with loose connective tissue, blood vessels, lymphatic vessels, and nerves. The mucous membrane, or endometrium, is lined by columnar ciliated secretory epithelium that undergoes cycles of proliferation, differentiation, and breakdown every 28 days in preparation for embryo implantation. The arterial blood supply to the female reproductive tract is provided by branches of the hypogastric and ovarian arteries from the abdominal aorta. The veins correspond with the arteries and end in the uterine plexuses. The nerves are derived from the hypogastric and ovarian plexuses and from the third and fourth sacral nerves.
The breast consists of glandular tissue organized in lobes connected by fibrous tissue, with fat deposits interspersed between the lobes (see Figure 9–1). The mammary lobes themselves are made of lobules connected by connective tissue, blood vessels, and ducts. The lobules consist of a cluster of rounded alveoli, which open into excretory lactiferous ducts and unite to form larger ducts made of longitudinal and transverse elastic fibers. These ducts converge toward the areola, beneath which they form ampullar dilatations, which serve as reservoirs for the milk. The arterial blood supply to the breast is derived from the thoracic branches of the axillary, the intercostal, and the internal mammary arteries. The veins drain into the axillary and internal mammary veins.
Gonadotropin Regulation of Ovarian Function
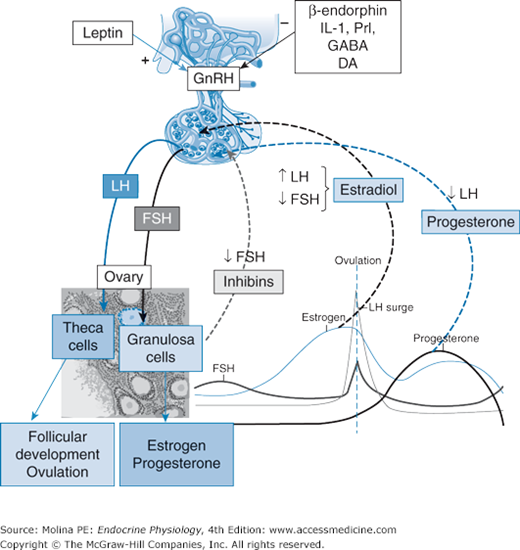
![]() Pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates pulsatile pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). As already mentioned in Chapter 8, GnRH secretion is regulated by dopamine, serotonin, β-endorphin, and norepinephrine. Both FSH and LH bind to G protein–coupled receptors, causing activation of the Gα-stimulatory (Gαs) subunit, which leads to cyclic 3′,5′-adenosine monophosphate (cAMP)-mediated stimulation of steroidogenic events (described in Chapters 6 and 8) culminating in ovarian production of estradiol and progesterone; the 2 principal hormones involved in the regulation of ovarian function and control of the reproductive cycle (Figure 9–2). The variations in pulsatile release of the gonadotropins result in a cyclic response of ovarian function. Each cycle lasts 28 days and can be divided into 2 phases (follicular and luteal) of 14 days each.
Pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates pulsatile pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). As already mentioned in Chapter 8, GnRH secretion is regulated by dopamine, serotonin, β-endorphin, and norepinephrine. Both FSH and LH bind to G protein–coupled receptors, causing activation of the Gα-stimulatory (Gαs) subunit, which leads to cyclic 3′,5′-adenosine monophosphate (cAMP)-mediated stimulation of steroidogenic events (described in Chapters 6 and 8) culminating in ovarian production of estradiol and progesterone; the 2 principal hormones involved in the regulation of ovarian function and control of the reproductive cycle (Figure 9–2). The variations in pulsatile release of the gonadotropins result in a cyclic response of ovarian function. Each cycle lasts 28 days and can be divided into 2 phases (follicular and luteal) of 14 days each.
Figure 9–2.
Hypothalamic-pituitary-ovarian axis. Gonadotropin synthesis and release and differential expression are under both positive and negative feedback control by ovarian steroid and peptide hormones. Ovarian hormones can decrease gonadotropin release both by modulating gonadotropin-releasing hormone (GnRH) pulse frequency from the hypothalamus and by affecting the ability of GnRH to stimulate gonadotropin secretion from the pituitary itself. Estradiol enhances luteinizing hormone (LH) and inhibits follicle-stimulating hormone (FSH) release, whereas inhibins A and B (gonadal glycoprotein hormones) reduce FSH secretion. After ovulation, ovarian progesterone production predominates. Progesterone increases hypothalamic opioid activity and slows GnRH pulse secretion, favoring FSH production and decreasing LH release. Inhibin B peaks early in the follicular phase, whereas inhibin A peaks in the midluteal phase. The increasing inhibin B levels in the midfollicular phase act at the pituitary gonadotroph to offset activin signaling and suppress FSH biosynthesis from early follicular phase levels. The decrease in inhibin A at the end of the luteal phase creates an environment in which FSH levels can again increase. GABA, γ-aminobutyric acid; DA, dopamine; IL-1, interleukin 1; NE, norepinephrine; NPY, neuropeptide Y; Prl, prolactin.
During the follicular phase, FSH stimulates follicular recruitment and growth and estrogen synthesis. Before the selection of the follicle for ovulation, granulosa cells are responsive only to FSH. As follicular maturation progresses, the coupling between FSH receptor stimulation and the activation of adenylate cyclase becomes more and more efficient, leading to a steady increase in cAMP production. The accumulation of FSH-induced cAMP results in upregulation of LH receptors, allowing LH to act as a surrogate for FSH in granulosa cells. Low gonadotropin levels (especially FSH) lead to granulosa cell death and follicular atresia.
LH is responsible for ovulation and corpus luteum formation and for progesterone and estrogen production by the corpus luteum. Activation of the LH receptors in theca cells stimulates androstenedione production, providing the substrate for the enzymatic conversion to 17β-estradiol that is mediated by the enzyme aromatase in granulosa cells.
Ovarian Hormone Synthesis
Ovarian production of steroid hormones (progesterone, estrogen, and testosterone) and peptide hormones (inhibins) varies throughout the ovarian cycle. The production and secretion rates of the principal ovarian steroid hormones are summarized in Table 9–1.
| Production/Secretion rate (mg/d) | ||
|---|---|---|
| Hormone | Follicular | Luteal |
| Progesterone | 2/1.7 | 25/24 |
| Estradiol | 0.09/0.08 | 0.25/0.24 |
| Estrone | 0.11/0.08 | 0.26/0.15 |
| Androstenedione | 3.2/2.8 | NC |
| Testosterone | 0.19/0.06 | NC |
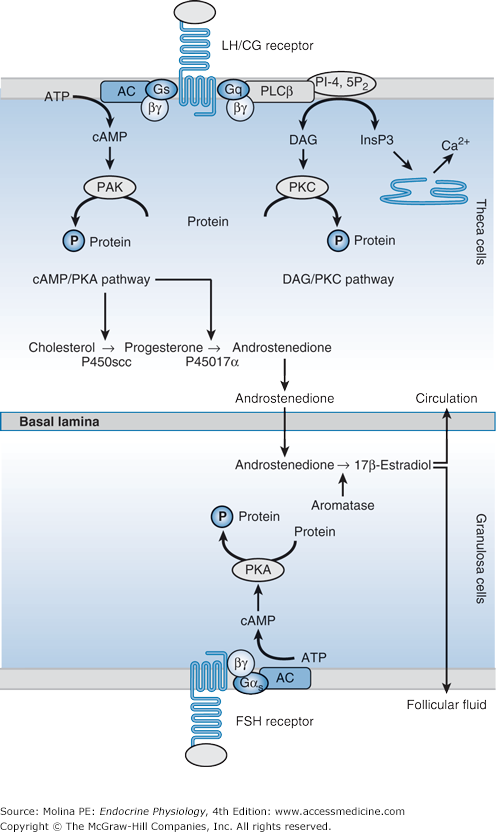
![]() The production of estrogen involves coordinated enzymatic activities between the theca and granulose cells of the ovarian follicle (Figure 9–3). Theca cells express the enzymes necessary to convert cholesterol to androgens (mainly androstenedione) but lack the enzymes necessary to convert androgens to estradiol. Granulosa cells can convert androgens to estradiol, and they can produce progesterone, but they are unable to synthesize androgens. Thus, theca cell–produced androgens are aromatized to estradiol by granulosa cells (see Figure 9–3). More than 95% of circulating estradiol is directly secreted by the ovaries, with a smaller contribution from peripheral conversion of estrone to estradiol in premenopausal women.
The production of estrogen involves coordinated enzymatic activities between the theca and granulose cells of the ovarian follicle (Figure 9–3). Theca cells express the enzymes necessary to convert cholesterol to androgens (mainly androstenedione) but lack the enzymes necessary to convert androgens to estradiol. Granulosa cells can convert androgens to estradiol, and they can produce progesterone, but they are unable to synthesize androgens. Thus, theca cell–produced androgens are aromatized to estradiol by granulosa cells (see Figure 9–3). More than 95% of circulating estradiol is directly secreted by the ovaries, with a smaller contribution from peripheral conversion of estrone to estradiol in premenopausal women.
Figure 9–3.
Theca and granulosa cells coordinate the production of estrogen. The secretion of estradiol by the dominant follicle requires cooperation between theca cells, which synthesize androstenedione and testosterone, and granulosa cells of mature follicles, which convert androgens to estradiol and estrone. Androgen synthesis in theca cells results from the activity of 3 enzymes: cholesterol side-chain cleavage (P450scc), 17α-hydroxylase-lyase (P450C17), and 3β-hydroxysteroid dehydrogenase (3β-HSD). Luteinizing hormone (LH) induces steroidogenic acute regulatory protein (StAR), which allows the entry of cholesterol into mitochondria. In granulosa cells, the enzyme 17β-hydroxysteroid dehydrogenase transforms androstenedione into testosterone in follicles from the primary stage. In mature follicles, follicle-stimulating hormone (FSH) stimulates the activity of aromatase, which transforms testosterone into 17β-estradiol. AC, adenylate cyclase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; DAG, diacylglycerol; InsP3, inositol 1,4,5-trisphosphate; PI-4,5P2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; PLCβ, phospholipase Cβ.
Female androgens are derived from the adrenal glands (dehydroepiandrosterone and androstenedione), from the ovaries (androstenedione and testosterone), and from peripheral conversion of androstenedione and dehydroepiandrosterone to testosterone. Ovarian androgen secretion parallels that of estrogen throughout the menstrual cycle, whereas adrenal androgen production does not fluctuate throughout the menstrual cycle. Most of the circulating testosterone in females is derived from the peripheral conversion of androstenedione by 17β-hydroxysteroid dehydrogenase. The conversion of testosterone to dihydrotestosterone in peripheral tissues is limited in females because of higher levels of sex hormone-binding globulin in females than in males, as well as by the peripheral conversion to estrogen by aromatase, protecting females from virilization by dihydrotestosterone.
The preovulatory LH surge results in luteinization of granulosa and theca cells, altering the steroidogenic pathway so that progesterone becomes the primary steroid hormone produced by each of these cell types after luteinization. The changes leading to the ability to produce progesterone include increased expression of the enzymes involved in the conversion of cholesterol to progesterone (cholesterol side-chain cleavage cytochrome P450 complex and 3β-hydroxysteroid dehydrogenase) and decreased expression of the enzymes that convert progesterone to estrogens (17α-hydroxylase cytochrome P450 and aromatase cytochrome P450).
![]() Inhibin production by granulosa cells of mature follicles is regulated by FSH and LH, and by local factors such as growth factors (epidermal, transforming, and insulin-like) and hormones (androstenedione, activin, and follistatin) in an autocrine and paracrine way. In clinical practice, inhibin B is a good marker of granulosa cell function under the control of FSH, whereas inhibin A is a marker of corpus luteum function under the control of LH. Inhibins contribute to the regulation of LH and FSH release through endocrine feedback regulation at the anterior pituitary.
Inhibin production by granulosa cells of mature follicles is regulated by FSH and LH, and by local factors such as growth factors (epidermal, transforming, and insulin-like) and hormones (androstenedione, activin, and follistatin) in an autocrine and paracrine way. In clinical practice, inhibin B is a good marker of granulosa cell function under the control of FSH, whereas inhibin A is a marker of corpus luteum function under the control of LH. Inhibins contribute to the regulation of LH and FSH release through endocrine feedback regulation at the anterior pituitary.
Activin production by the granulosa cells changes during folliculogenesis and its effects are probably limited to paracrine action on granulosa cells. Activin promotes granulosa cell proliferation, upregulates FSH receptor expression on granulosa cells, and modulates steroidogenesis in both granulosa and theca cells. In the pituitary-ovarian axis, activin is a physiologic antagonist to inhibin and specifically stimulates pituitary FSH synthesis and secretion.
Activin-binding protein, follistatin, is a product of granulosa cells. Its basal expression increases with differentiation of granulosa cells. Its function is to neutralize the effect of activin on steroid production. The physiologic endocrine role of follistatin is not completely understood, but its effects are most likely autocrine or paracrine on ovarian steroidogenesis.
Ovarian Cycle
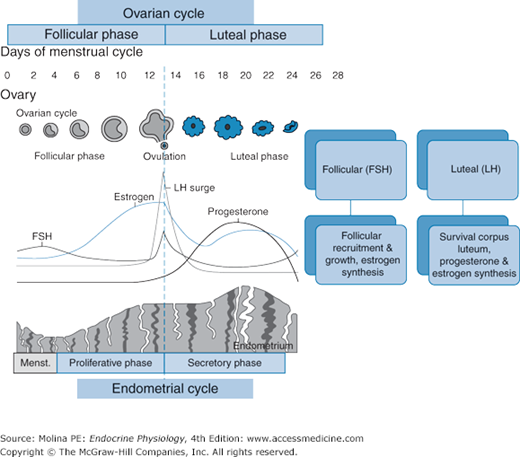
Figure 9–4.
Hormonal events during the ovarian and endometrial cycles. Plasma concentrations of inhibins, estrogen, progesterone, luteinizing hormone, and follicle-stimulating hormone (FSH) during the human menstrual cycle correspond to proliferative and secretory changes in the endometrium and to follicular development and ovulation.
The follicular phase begins on day 1 of the cycle, the first day of menses, and corresponds to the growth and development of a dominant follicle. Throughout the reproductive life span of the woman (from puberty to menopause), a single mature oocyte is produced each month. Most of the human oocytes (germ cells) present during uterine development are destined to undergo apoptosis, or programmed cell death. Only follicles that are responsive to FSH stimulation (approximately 350) will enter the final stage of development and progress to ovulation. At mid cycle (day 14), the rising levels of estrogen stimulate a surge in LH release, which stimulates ovulation 24–36 hours later.
The luteal phase begins after ovulation, with the reorganization of the remnants of the ovulatory follicle and the formation of the corpus luteum (see Figure 9–4). The corpus luteum is a transient endocrine organ that produces progesterone, and to a lesser extent estradiol and inhibin A. The corpus luteum is under LH regulation. The luteal phase ends when there is no fertilization and placental-mediated survival of the corpus luteum discussed below.
Gonadotropin release is under negative and positive feedback regulation by estradiol, progesterone, and inhibins A and B. Progesterone and 17β-estradiol act both in the hypothalamus and the pituitary, and inhibins act at the level of the pituitary (see Figure 9–2). The contributions of these ovarian hormones vary according to the stage of the ovarian cycle.
During this phase, the dominant follicle produces high concentrations of 17β-estradiol and inhibin B. Although initially estradiol exerts negative feedback on FSH and LH release, as concentrations of estradiol increase, toward the end of the follicular phase, a switch from negative to positive feedback occurs. High estradiol levels in the hypothalamus and pituitary lead to low-amplitude, high-frequency pulses (every 90 minutes) of LH, resulting in a midcycle LH surge. The estradiol-mediated stimulation of the LH surge results from an increased responsiveness of gonadotropic cells to GnRH (following exposure to increasing estradiol levels), an increase in GnRH receptor number, and a GnRH surge, triggered by the effect on the hypothalamus of increasing estradiol concentrations (see Figures 9–2 and 9–4). Inhibin B levels rise during the follicular phase and decrease immediately before the LH peak, with a brief surge occurring 2 days after ovulation. Inhibin A levels increase in the late follicular phase to reach a peak concentration on the day of the LH and FSH surge. The concentration then falls briefly before rising to reach a maximum concentration during the midluteal phase.
The midcycle surge in LH levels induces ovulation, resumption of meiosis, and promotes the formation and survival of the corpus luteum during the luteal phase. During the luteal phase, high circulating concentrations of progesterone (produced by the corpus luteum) suppress the frequency and the amplitude of LH release, resulting in an overall decrease in LH by blocking the surges of GnRH, downregulating pituitary GnRH receptor expression, and decreasing gene expression of the α- and β-subunits of both LH and FSH. Thus, negative feedback regulation by progesterone during the luteal phase prevents a second LH surge. The marked suppression of GnRH and LH pulse frequency achieved by high progesterone levels during the luteal phase allows enrichment of gonadotroph FSH levels. Inhibin B levels remain low during the luteal phase. Inhibin A is secreted by the granulosa cells during the luteal phase, and its concentration falls during luteal regression synchronously with estradiol and progesterone, remaining low during the early follicular phase.
Unless the corpus luteum is stimulated by human chorionic gonadotropin (hCG), a placental hormone (described later), it regresses. Regression or lysis of the corpus luteum and the associated decrease in progesterone levels leads to an increase in FSH release toward the beginning of the next ovarian cycle. During the luteal-follicular transition, following the midcycle rise in FSH secretion, the inhibin B concentration rises and peaks 4 days after the peak FSH concentration is reached.
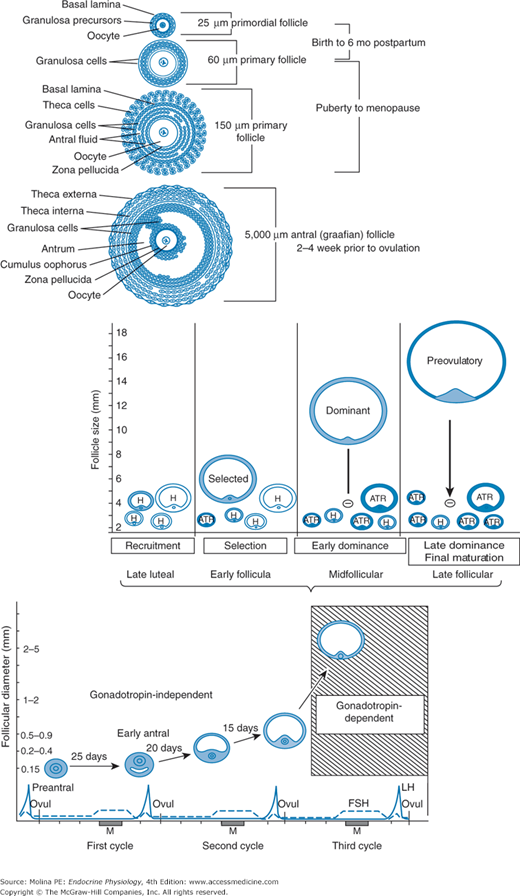
![]() Unlike the fetal testis, the fetal ovary begins germ cell development (oogenesis) early in fetal life. During early intrauterine development (15 weeks), primordial germ cells (oogonia) proliferate and migrate to the genital ridge. On their arrival in the fetal ovary, some of the oogonia continue mitotic proliferation and some begin to undergo apoptosis (Figure 9–5). Some of these oogonia begin (but do not complete) meiosis and become oocytes. These cells have 2 X chromosomes. By 6 months postpartum, all oogonia have been converted to oocytes. At or near birth, the meiotic process is arrested at prophase of the first meiotic division. The oocytes remain arrested in the diplotene stage of the first meiotic prophase until they are recruited to grow and mature (by FSH) to produce an ovum or they undergo apoptosis. During the first days of postnatal life, the oocytes recruit somatic follicular cells, which are organized into a finite number of “resting” primordial follicles. Primordial follicles are composed of an outer layer of granulosa cells and a small oocyte, both enveloped in a basal lamina. The pool of primordial follicles in the female ovary reaches its maximum number at approximately 20 weeks of gestational age and then decreases in a logarithmic fashion throughout life until complete depletion occurs during menopause. When reproductive life is initiated, less than 10% of the primordial follicles are left.
Unlike the fetal testis, the fetal ovary begins germ cell development (oogenesis) early in fetal life. During early intrauterine development (15 weeks), primordial germ cells (oogonia) proliferate and migrate to the genital ridge. On their arrival in the fetal ovary, some of the oogonia continue mitotic proliferation and some begin to undergo apoptosis (Figure 9–5). Some of these oogonia begin (but do not complete) meiosis and become oocytes. These cells have 2 X chromosomes. By 6 months postpartum, all oogonia have been converted to oocytes. At or near birth, the meiotic process is arrested at prophase of the first meiotic division. The oocytes remain arrested in the diplotene stage of the first meiotic prophase until they are recruited to grow and mature (by FSH) to produce an ovum or they undergo apoptosis. During the first days of postnatal life, the oocytes recruit somatic follicular cells, which are organized into a finite number of “resting” primordial follicles. Primordial follicles are composed of an outer layer of granulosa cells and a small oocyte, both enveloped in a basal lamina. The pool of primordial follicles in the female ovary reaches its maximum number at approximately 20 weeks of gestational age and then decreases in a logarithmic fashion throughout life until complete depletion occurs during menopause. When reproductive life is initiated, less than 10% of the primordial follicles are left.
Figure 9–5.
Follicle growth and development. Folliculogenesis, or formation of the dominant follicle, consists of 2 stages: the gonadotropin-independent (preantral) period and the gonadotropin-dependent (antral or graafian) period. Primordial follicular growth up to the antral stage occurs during fetal life and infancy and is gonadotropin independent. The final developmental phase of follicular growth, in which antral follicles are protected from apoptosis, begins approximately 85 days before ovulation. One dominant follicle is recruited in the luteal phase of the cycle preceding ovulation. Atr, atretic; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Folliculogenesis, or formation of the dominant follicle, consists of 2 stages: the gonadotropin-independent (preantral) period and the gonadotropin-dependent (antral or graafian) period. Primordial follicular growth up to the antral stage (up to 0.2 mm) occurs during fetal life and infancy and is gonadotropin independent (see Figure 9–5). Primary follicles are formed when the flattened epithelial cells become cuboidal and undergo mitosis. The antral follicle growth phase is characterized by granulosa cell proliferation, expression of FSH and steroid hormone receptors, and association of the theca cells with the growing follicle and granulosa cells, leading to formation of the secondary follicles. Tertiary follicles are formed following further theca cell hypertrophy and development. Their antrum is filled with estrogen-rich fluid, and the theca interstitial cells start to express FSH and LH receptors. The mechanisms that trigger initiation of follicular growth are still not completely understood, but are thought to involve bidirectional communication between germ and somatic cells through gap junctions and paracrine factors, including cytokines and growth factors (insulin-like growth factor 1 [IGF-1], epidermal and fibroblast growth factors, and interleukin 1β). When follicles reach a size of 2–5 mm, approximately 50% enter the selection growth phase and are rescued from apoptosis. This final developmental phase of follicular growth begins approximately 85 days before ovulation in the luteal phase of the cycle preceding ovulation (see Figure 9–5).
![]() During this gonadotropin-dependent growth phase, follicles grow exponentially, and FSH stimulates estrogen production from granulosa cells, follicular fluid formation, cell proliferation, and LH receptor expression in the dominant follicle. Selection of a dominant follicle is dictated by sensitivity to FSH action, which is locally modulated by antimüllerian hormone (AMH).
During this gonadotropin-dependent growth phase, follicles grow exponentially, and FSH stimulates estrogen production from granulosa cells, follicular fluid formation, cell proliferation, and LH receptor expression in the dominant follicle. Selection of a dominant follicle is dictated by sensitivity to FSH action, which is locally modulated by antimüllerian hormone (AMH).
AMH or müllerian-inhibiting substance discussed in Chapter 8 as it pertains to sex differentiation in the male embryo, is a peptide growth factor and member of the large transforming growth factor beta family of growth and differentiation factors. AMH is expressed in the granulosa cells of the recruited primordial follicles, and continues to be expressed in the growing follicles that have undergone recruitment from the primordial follicle pool but that have not been selected for dominance. This pattern of expression suggests an important role in the regulation of both the number of growing follicles and their selection for ovulation. Because the number of growing follicles is correlated to the size of the primordial follicle pool size, a marker such as AMH that reflects all follicles that have made the transition from the primordial follicle pool to the growing pool has been proposed to be a good indirect marker of ovarian reserve. FSH and inhibin B levels have also been proposed to serve as predictors of the ovarian reserve.
The average time between primary follicle development and ovulation is 10–14 days (see Figure 9–5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree