Extrathoracic Revascularization (Carotid-Carotid, Carotid-Subclavian Bypass and Transposition)
Edward Y. Woo
Scott M. Damrauer
DEFINITION
Extrathoracic revascularization, including carotid-subclavian and carotid-carotid bypass, involves the bypass of the proximal great vessels outside of the chest. Initially described for treatment of cerebrovascular and upper extremity occlusive disease, these procedures are commonly now employed to create a proximal seal zone for endovascular treatment of thoracic aortic disease by “debranching” the aortic arch.
Carotid-subclavian bypass is accomplished by inserting a graft conduit between the mid-common carotid artery to the ipsilateral subclavian artery.
Subclavian artery transposition is a potential alternative to carotid-subclavian bypass requiring division of the subclavian artery proximal to the vertebral artery and transposing it to the ipsilateral common carotid artery. It is an efficient way to revascularize the subclavian artery without the use of prosthetic conduit.1
Carotid-carotid bypass provides flow from one common carotid artery to the contralateral common carotid artery.
When carotid-carotid bypass is performed in a right-to-left manner and in conjunction with carotid-subclavian bypass, the blood flow to the left brain can be preserved while allowing for extension of the proximal thoracic endovascular aortic repair (TEVAR) seal zone to cover the left common carotid artery.
PATIENT HISTORY AND PHYSICAL FINDINGS
The history should focus on neurologic symptoms that may indicate the presence of symptomatic cerebrovascular disease. Previous head and neck or carotid surgery should be noted, as well as a history of head, neck, or upper chest region external beam radiation therapy, as these may significantly increase the complexity of the procedure.
The directed physical exam should be focused on detection of underlying vascular disease that may complicate planned intervention. Bilateral upper extremity blood pressures should be obtained; a difference of greater than 10 mmHg indicates the potential presence of preexisting occlusive disease. Likewise, the presence of carotid bruits, delayed carotid upstrokes, or abnormal upper extremity pulses suggests arterial occlusive disease that should be delineated prior to extrathoracic reconstruction or bypass of the great vessels.
Special attention should be directed toward the cranial nerves and voice, especially in patients with prior cervical surgical procedures. Indirect laryngoscopy should be performed preoperatively in patients with hoarseness or in whom a preexisting vocal cord or cranial nerve deficit has been noted.
Neck mobility and the presence of cervical spinal disease should be assessed, as neck extension and rotation is essential for adequate operative exposure. Patients with relative neck immobility may be poorly suited for these procedures.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Carotid duplex scanning should be used to identify patients with carotid artery stenosis prior to planned bypass procedures. Failure to identify and address stenoses at the carotid bifurcation may lead to postoperative steal phenomenon and neurologic sequelae. Manipulation of the diseased carotid artery may also increase the risk of periprocedural stroke. In these circumstances, concomitant or staged carotid intervention may be warranted.
Computed tomographic (CT) angiography of the aortic arch and proximal carotid arteries provides the anatomic detail necessary to safely perform carotid-subclavian bypass, subclavian artery transposition, or carotid-carotid bypass. This study is complementary to duplex scanning, as it provides anatomic rather than hemodynamic assessment and images vessels equally well inside and outside the chest. CT scanning also visualizes the course of the subclavian artery in relationship to the clavicle, as its course may also be distorted by a large arch aneurysm.
SURGICAL MANAGEMENT
Preoperative Planning
Neuromonitoring is a useful adjunct to ensure adequacy of cerebral perfusion from the contralateral cerebral circulation when the ipsilateral common carotid artery is clamped. Numerous modalities exist for neuromonitoring, including electroencephalography (EEG), transcranial Doppler, near-infrared spectroscopy, and stump pressure measurement. An indwelling carotid shunt may be placed to improve ipsilateral blood flow when monitoring indicates cerebral perfusion is inadequate. This problem occurs infrequently, as only the common carotid is occluded, but preparations should be made for shunting procedures when indicated. Alternatively, as with carotid endarterectomy (CEA), in the absence of neuromonitoring, shunts may be placed prophylactically to preserve carotid flow in all cases.
Invasive continuous arterial pressure monitoring is routinely employed, with line placement dictated by the laterality of the procedure. Keeping in mind the potential need to occlude the subclavian artery for the reconstruction, the arterial line should be placed in the contralateral limb or in a femoral artery.
Positioning
The patient is positioned supine with the head rotated away from the operative side. A pneumatic pillow is placed under the shoulders to allow for neck extension. Careful attention must be paid to achieve maximum neck extension while still supporting the occiput. The bed is placed in a semi-Fowler’s position to reduce venous pressure and minimize bleeding.
For carotid-carotid bypass, the head is positioned midline to facilitate bilateral dissection.
TECHNIQUES
CAROTID-SUBCLAVIAN BYPASS
Exposure of the Subclavian Artery
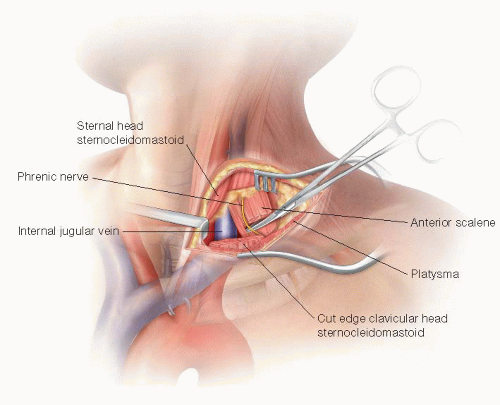
The incision is extended from the lateral aspect of the clavicular head of the sternocleidomastoid (SCM) muscle laterally across the supraclavicular fossa. This is further developed through the subcutaneous tissue and platysma with electrocautery. If the external jugular vein is encountered, it should be ligated and divided.
Sufficient clavicular head of the SCM is divided to allow for adequate medial exposure. Up to one-half of the sternal head of the SCM can also be divided if also needed, but this is rarely necessary. The scalene fat pad is then visualized and divided. It is preferable to divide this near its inferior border so that most of the fat pad can be preserved and reclosed to cover the reconstruction. Care must be taken to identify and preserve the phrenic nerve as it courses over the anterior scalene muscle deep to the fat pad. The thoracic duct is easily identified. If it is injured or in the way, it should be ligated to prevent significant morbidity from a postoperative lymphatic leak.
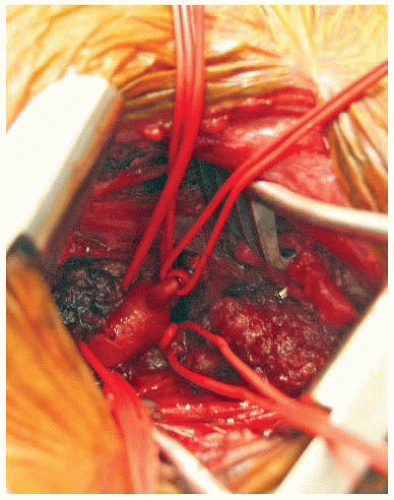
Once the fat pad has been mobilized, the anterior scalene muscle is divided to reveal the underlying subclavian artery (FIG 1). It is best to divide the muscle slowly and in layers to prevent injury to the underlying vessel. The subclavian artery is dissected circumferentially and controlled with vessel loops. Care must be taken when manipulating this vessel, as the subclavian artery is significantly more fragile and prone to injury than lower extremity arteries of comparable diameter (e.g., femoral or popliteal). Depending on the method of reconstruction and location of the planned anastomosis, the thyrocervical trunk, inferior mammary, and vertebral arteries may need to be controlled separately (FIG 2).
Exposure of Carotid Artery
In the medial aspect of the wound, the lateral border of the internal jugular vein is identified and sharply defined. The vein is retracted posteriorly and the carotid sheath is entered from the lateral posterior margin. Care must be taken to identify the vagus nerve early, as its usual posterior position places it immediately in the field of dissection as the sheath is opened from this approach.
The common carotid artery is dissected circumferentially (FIG 3). Only 5 cm of artery needs to be isolated in order to obtain control and perform the anastomosis. The dissection should stay proximal to the carotid bulb, which minimizes risk of cerebral embolization and injury to more proximal nerves.
Bypass
Either Dacron or polytetrafluoroethylene (PTFE) can be used as conduits for extrathoracic bypass with no difference in outcomes.2 Autogenous vein grafts should be avoided, however, as their long-term patency is inferior to prosthetic in this location.3 We favor Dacron given the size ranges available and the relative resistance of the graft to kinking over the short distance of the reconstruction.
Prior to arterial clamping, systemic anticoagulation is achieved with intravenous heparin administration. The activated clotting time (ACT) should be monitored and additional heparin administered throughout the procedure to maintain adequate anticoagulation.
The subclavian anastomosis is performed first. Arterial control (vessel loops or clamps) is obtained and the vessel is opened with a longitudinal arteriotomy. The anastomosis should be fashioned in the position most favorable to the planned graft. The graft is beveled and trimmed so that the graft lies at an approximately 60-degree angle to the artery. A running Prolene suture is used to perform the anastomosis with completion of the back wall first. Once the anastomosis is complete, the graft is clamped and flow restored to the arm by unclamping the subclavian artery. It is useful to flush through the graft to remove any debris prior to opening to the arm. The graft should also be flushed with heparinized saline and clamped near the anastomosis to avoid any thrombosis of the stagnant blood column within the graft. If repairs are needed, control is restored and pledgeted sutures are used to avoid injury to the fragile artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree