|
Carotid Body Tumor |
Jugulotympanic |
Retroperitoneal Intra-abdominal |
Cauda Equina/Filum Terminale |
Synonyms |
Chemodectoma, nonchromaffin paraganglioma |
Glomus tumor, glomus jugulare, glomus tympanicum |
Extra-adrenal paraganglioma |
– |
Location |
Neck, at the bifurcation of the carotid artery; localized deep to the anterior border of sternocleidomastoid muscle, just below the angle of mandible; may be attached to the arteries or even encase them |
Middle ear, external meatus 85%, parapharyngeal space, base of the skull; intracranial extension possible |
Along the sympathetic trunk; commonly in retroperitoneum; can arise from organs of Zuckerkandl visceral involving urinary bladder, gall bladder |
Intradural, or extradural; attached to filum terminale or a nerve root |
Incidence |
85% of head and neck paragangliomas |
Most common tumor of the middle ear |
90% associated with sympathetic nervous system |
Very uncommon |
Average age |
Wide age range but common in fifth decade |
Fifth decade |
Third to fifth decade |
Third to fifth decade |
Gender predilection |
None; but common in females at higher altitude |
Common in females; M:F, 1:5 |
None |
Slight increase in males |
Radiologic findings |
Homogeneous hypervascular, well-delineated mass lesion at the carotid artery bifurcation by carotid arteriogram |
Soft tissue mass; bone erosion |
Mass on CT scan |
Mass on CT scan, MRI, blockage on myelogram |
Association with other paragangliomas and syndromes |
May be associated with jugulotympanic paragangliomas, a part of Carney triad (GIST, pulmonary chondroma, and carotid body tumor); may be associated with pheochromocytoma, or MEN syndrome |
May be associated with carotid body paraganglioma; can be part of familial multifocal head and neck paragangliomas as an autosomal dominant trait |
None |
None |
Presenting symptoms |
Painless, slowly enlarging neck mass, located below the angle of the mandible; deep to the anterior border of sternocleidomastoid muscle; vertically fixed but movable horizontally; may be pulsatile |
Tinnitus; aural pulsations; conduction-type hearing loss; ear fullness; pain; otorrhea; vertigo; dizziness; facial palsy; bulging of tympanic membrane; tumor may fill the middleear cavity and extend into external auditory canal or extend into cranial cavity |
Back pain; palpable mass; symptoms due to secretion of norepinephrine |
Lower back pain, radicular pain or sciatica is common; sensory-motor deficits include paraplegia and sphincter disturbances |
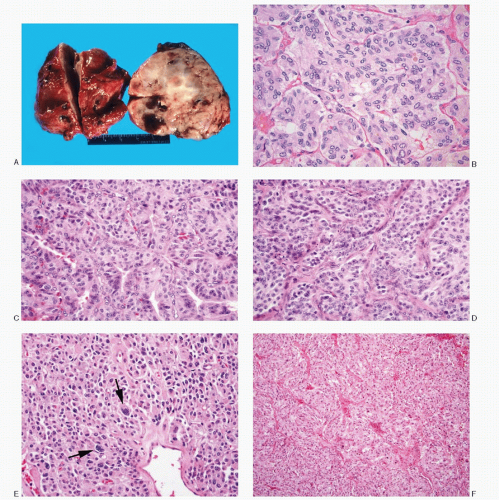
Gross pathology |
Encapsulated, well circumscribed; ovoid, rubbery to firm; redpink to tan-gray |
Polypoid, red fragile mass; bleed profusely to touch |
3-20 cm; well-defined mass, solid cut surface; hemorrhage and cystic change frequent; usually solitary |
Generally egg shaped or sausage shaped; encapsulated; dark red, attached to filum terminale or a nerve root; size 2-4 cm |
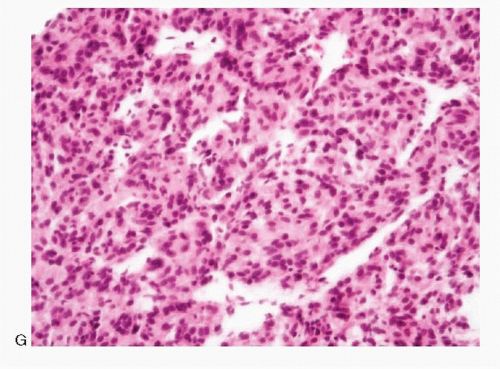
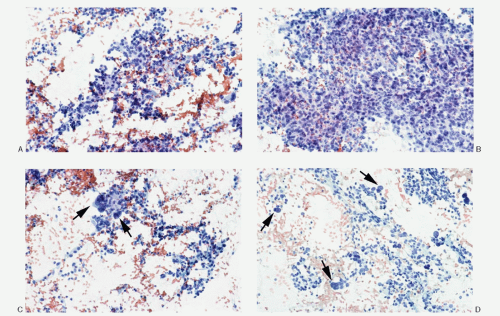
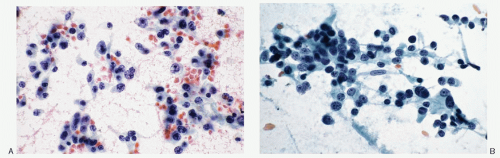
Histology |
Typical alveolar or nesting pattern (Zellballen); can be trabecular, or solid with diffuse growth pattern; monomorphic to pleomorphic; scant stroma |
Typical nesting pattern; may show marked stromal fibrosis, lacking the typical pattern |
Typical alveolar or nesting pattern (Zellballen); can be trabecular, or solid with diffuse growth pattern; monomorphic to pleomorphic; scant stroma; prominent vascularity; no attached remnants of adrenal tissue |
Typical alveolar or nesting pattern or solid growth pattern; ganglionic differentiation frequent; may express cytokeratin |
Differential diagnoses |
Medullary thyroid carcinoma
Malignant melanoma
Alveolar soft-part sarcoma
Malignant lymphoma
Metastatic carcinoma |
Hemangiopericytoma
Pituitary adenoma
Meningioma, small cell type
Malignant lymphoma
Plasmacytoma
Olfactory neuroblastoma
Middle ear adenoma |
Adrenocortical carcinoma
Renal cell carcinoma
Hepatocellular carcinoma
Metastatic poorly differentiated carcinoma
Malignant melanoma
Malignant lymphoma
Soft tissue tumors |
Hemangiopericytoma
Ependymoma
Chondrosarcoma
Malignant lymphoma
Plasmacytoma
Meningioma |



 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access