Exposure and Open Surgical Reconstruction in the Chest: The Thoracoabdominal Aorta
Germano Melissano
Efrem Civilini
Enrico Rinaldi
Roberto Chiesa
DEFINITION
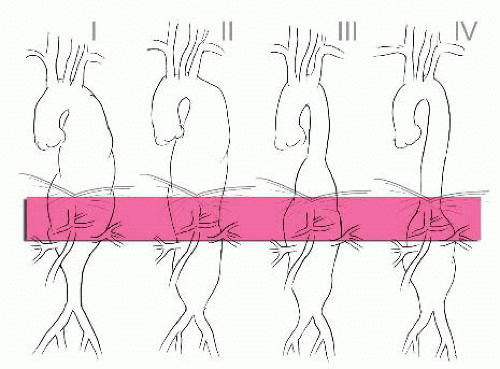
A thoracoabdominal aortic aneurysm (TAAA) involves the aorta at the diaphragmatic crura and extends variable distances proximally and/or distally from this point (FIG 1).1 TAAAs can be classified in terms of their causes, the two most common being medial degeneration and dissection.
Open treatment of TAAAs consists of graft replacement with reattachment of the main aortic branches: The inclusion technique was introduced by S. E. Crawford in the 70s and refined by subsequent surgeons in the following decades. TAAA repair, especially in extensive aortic disease, is associated with greater operative risk than repair of other aortic segments. The main sources of morbidity are spinal cord (SC) ischemia and renal as well as respiratory and cardiac complications.
IMAGING AND OTHER DIAGNOSTIC STUDIES
To plan the best possible treatment strategy for each patient, our preferred modality is computed tomographic arteriography (CTA). The acquisition of computed tomography (CT) data in particular has benefited from spectacular progress, including multirow detectors, higher rotation and translation speeds with reduced scan times (single breath-hold), cardiac cycle synchronization, and better postprocessing capabilities.
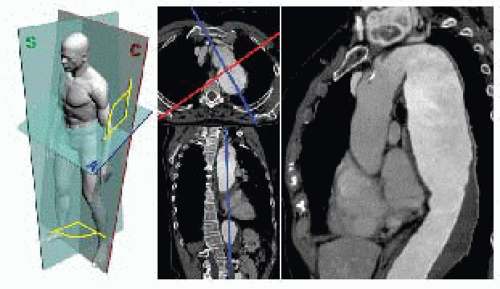
Digital Imaging and Communications in Medicine (DICOM) slices of adequate thickness (≤1 mm) should be postprocessed on a digital workstation using a multiplanar reformatting (MPR) tool to visualize a scan which angulation matches that of the aorta or the vessel under investigation. Postprocessing may be performed on a dedicated workstation (AquariusNet®, TeraRecon, Inc) or desktop computer with open source software (OsiriX and others) in a user-friendly and time/resources-efficient way (FIG 2).
Beyond analysis of aortic diameter and the extent of pathologic involvement, reformatted images are particularly useful for evaluating the presence, extension, and characteristics of dissection and thrombus, particularly at proposed sites of clamp placement and the infradiaphragmatic aorta when direct aneurysm cannulation is considered for distal aortic perfusion. The exact location and geometry of aortic branches is obtained to reveal possible anatomic variations or anomalies, which are particularly common at the level of the renal arteries and arch vessels. Vessel patency is also routinely evaluated; in particular, obstruction of the superior and inferior mesenteric artery and the hypogastric arteries and dominance of one vertebral artery are assessed.
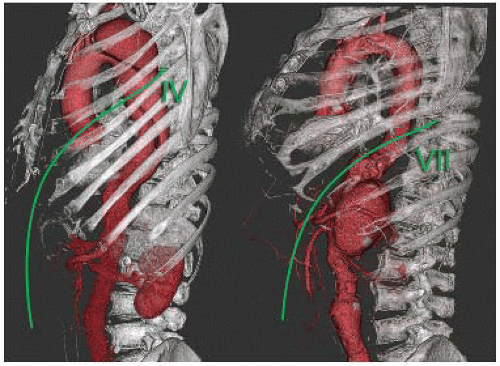
Three-dimensional rendering tools such as maximum intensity projection (MIP), volume rendering, surface rendering, and so forth produce realistic imaging of the anatomic structures that may expand anatomic understanding, including, for instance, the most appropriate intercostal space to perform thoracotomy (FIG 3).
Perioperative SC ischemia may precipitate paraparesis or paraplegia. Prior knowledge of the SC arterial supply informs both procedural planning and risk stratification.
Recent advances in imaging techniques, especially noninvasive techniques, increased the likelihood that patientspecific risk criteria may soon be recognized and be widely available4 (FIG 4).
SURGICAL MANAGEMENT
Preoperative Workup and Patient Optimization
Preoperative transthoracic echocardiography is a satisfactory noninvasive screening method to evaluate both valvular and biventricular function. Stress testing identifies patients who require coronary catheterization and possible intervention.5 Electrocardiographically (EKG) gated CT has recently emerged as a less invasive method of visualizing coronary anatomy. For severe, symptomatic coronary disease requiring percutaneous transluminal angioplasty prior to aneurysm repair, use of drug-eluting stents requiring prolonged double antiplatelet therapy should be avoided to reduce subsequent perioperative bleeding.
The use of estimated glomerular filtration rate (eGFR), rather than serum creatinine levels alone, is recommended to assess renal function.6 Based on the eGFR metric, chronic kidney disease has been shown to be a strong predictor of death following open or endovascular thoracic aneurysm repair, even in patients without other clinical evidence of preoperative renal disease.7
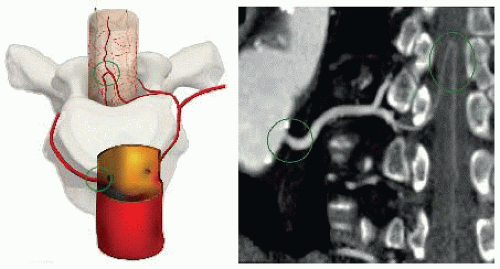 FIG 4 • Using a customized curve plan, the whole path of the arterial feeder to the spinal cord (arteria radicularis magna) can be visualized from the aorta to the anterior spinal artery. |
Pulmonary function evaluation with arterial blood gases and spirometry is used to evaluate the respiratory reserve of all patients undergoing open surgery of the descending aorta. In patients with a forced expiratory volume in 1 second (FEV1) of less than 1 L and a partial pressure of carbon dioxide (PCO2) greater than 45 mmHg, operative risk may be improved by cessation of cigarette smoking, treatment of chronic bronchitis (if present), weight loss, and participation in a supervised exercise program for a period of up to 6 months prior to surgery. However, in patients with aneurysm-related symptoms, this type of respiratory rehabilitation may not be practical or possible.
Positioning
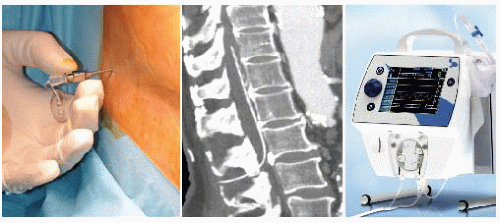
After inserting a cerebrospinal fluid drainage (CSFD)8 catheter into the subarachnoid space between L2 and L3 or L3 and L4 (FIG 5), the patient is turned to a right lateral decubitus position, with the shoulders at 60 degrees and the hips flexed back to 30 degrees.
Preparation should allow for access to the entire left thorax, abdomen, and both inguinal regions. Patient position is maintained with a moldable beanbag attached to a suction line for vacuum creation. A circulating water mattress is placed between the beanbag and the patient in order to modify body temperature as necessary (FIG 6).
TECHNIQUES
THORACO-PHRENO-LAPAROTOMY
The thoracic incision varies in length and level, depending on exposure requirements. Usually, the 5th, 6th, or 7th intercostal space is employed according to the aneurysm anatomy. The posterior section of the ribs is gently spread to reduce thoracic wall trauma and fractures; anterolaterally, the incision curves gently as it crosses the costal margin to minimize subsequent tissue necrosis. The pleural space is entered after single right lung ventilation is initiated. Monopulmonary ventilation is maintained throughout thoracic aorta replacement (FIG 7).
Paralysis of the left hemidiaphragm contributes significantly to postoperative respiratory failure; therefore, a limited circumferential rather than radial section of the diaphragm is routinely performed, sparing the phrenic center. Under favorable anatomic conditions, this approach reduces respiratory weaning time9 (FIG 8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree