Evaluation of Fibrosis in Renal Allograft
A. Brad Farris, III, MD
TERMINOLOGY
Definitions
Interstitial fibrosis (IF): Accumulation of collagen & related molecules in interstitium
Synonyms
Scarring
Sclerosis
EPIDEMIOLOGY
Natural History
Extent of IF predicts renal allograft outcome & may be considered a surrogate marker
Studies show reciprocal correlation between kidney function & IF extent
IF & tubular atrophy (TA) have been associated with
Cold ischemia time
Clinical & subclinical acute rejection
Preexisting donor damage
Degree of sensitization
Cyclosporine exposure
Renal calcifications
IF shows prognostic value in renal donor biopsies
↑ risk of adverse outcome at 6 months
1.9x greater prediction from age alone with Banff index for IF (ci score > 0)
Morphometric interstitial volume: Correlates with graft function at 1 year
ETIOLOGY/PATHOGENESIS
Histogenesis
Molecular mediators
Transforming growth factor (TGF)-β
Bone morphogenetic protein (BMP)
Platelet-derived growth factor (PDGF)
Hepatocyte growth factor (HGF)
Recent genomic approaches show altered molecular factors in IF
Cellular mediators
Epithelial cells
Fibroblasts/myofibroblasts & fibrocytes
Inflammatory cells: Lymphocytes, monocyte/macrophages, dendritic cells, mast cells
Endothelial cells
Epithelial-to-Mesenchymal Phenotype (EMP)
Chronically injured epithelial cells may undergo transition to mesenchymal cells
Process termed “epithelial-to-mesenchymal transition (EMT)” in past literature
Injured epithelium may change morphology and express mesenchymal-like markers, but the actual EMT process has not been directly observed in vivo
So-called “EMT” may simply reflect a change in protein expression rather than a true transition
Mesenchymal markers are not entirely specific, making research questionable (per a recent Banff conference and other publications)
Thus, “EMP” may be more appropriate since changes may simply be the observation of altered phenotype
CLINICAL IMPLICATIONS
Clinical Presentation
IF contributes to functional deterioration
IF/TA associated with transplant vasculopathy, ↑ serum creatinine, or transplant glomerulopathy implies poorer prognosis than IF/TA without additional lesions
Protocol biopsies to assess IF progression can be useful in demonstrating the baseline state of the allograft as well as stepwise changes that are occurring
e.g., protocol biopsies can be helpful in clinical trials, investigation of drug effectiveness, etc.
MICROSCOPIC FINDINGS
Qualitative Visual Assessment
Not all fibrosis is “equal” or “the same” in quality & quantity
“Early,” “young,” or “active” IF may have greater potential for remodeling
Broad scars: Pyelonephritis & infarcts can produce severe focal injury & parenchymal destruction
Diffuse, fine IF: Diffuse disease of glomeruli, tubules, & vessels
IF patterns may have different implications
“Striped,” patchy fibrosis described with calcineurin inhibitor use, possibly due to preferential medullary ray involvement
Chronic obstructive pattern: Atubular glomeruli, dilated tubules, & intratubular Tamm-Horsfall protein casts with interstitial extravasation
Inflammation in areas of IF has been noted to be an adverse risk factor for renal disease progression
IF & TA typically correlate (i.e., IF/TA)
TA may be profound in renal artery stenosis, with little or no accompanying IF
IF/TA (graded I-III based on the same cutoffs as ci) has effectively replaced the term “chronic allograft nephropathy (CAN)”
Subcapsular, perivascular, & periglomerular IF are typically not counted, but objective exclusionary criteria are lacking
IF assessment typically focuses on the cortex, but many emphasize that medullary fibrosis is also important
Quantitative Visual Assessment
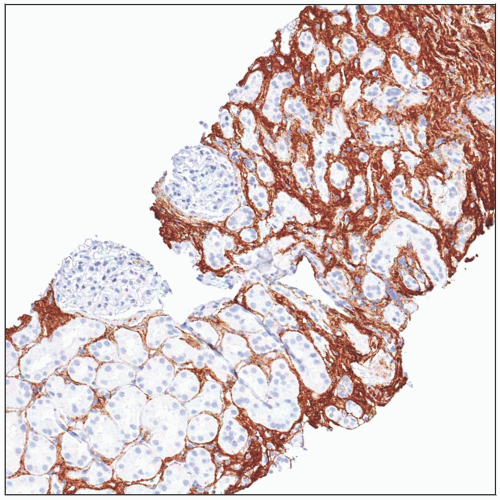
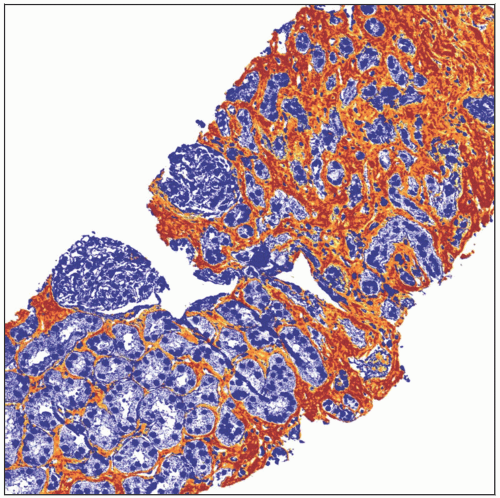
Most IF scoring systems (notably Banff) are based on a quantitation of % of cortical parenchyma involved
Banff IF scoring [termed “ci score”] uses following cutoffs
ci0: ≤ 5%, ci1: 6-25%, ci2: 26-50%, ci3: > 50%
Special stains