Dispersed Neuroendocrine System: an Overview
Min W. Lee
Sudha R. Kini
INTRODUCTION
Neuroendocrine neoplasia is an enigmatic family of tumors sharing a phenotype characterized by the simultaneous expression of multiple genes, which encode a wide variety of neuronal and endocrine traits (Rosai, 2011).
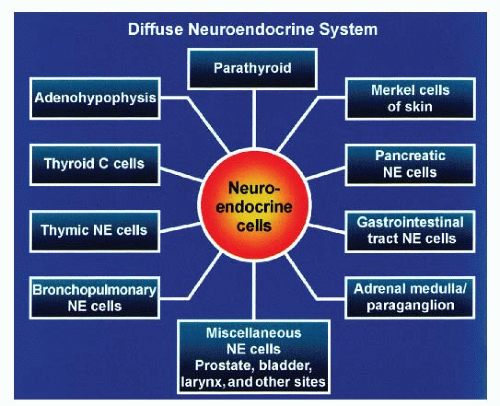
Neuroendocrine-programmed cells are distributed widely in the body regardless of their embryologic derivation (Fig. 1.1). Certain organs are entirely composed of neuroendocrine cells such as the pituitary gland and parathyroid glands. In other nonneuroendocrine organs, neuroendocrine cells are aggregated to form structures that can be identified microscopically with routine stains (H&E). Examples are adrenal medulla and pancreatic islets of Langerhans. Neuroendocrine cells are also diffusely dispersed in the epithelium mostly of endodermalderived organs such as the salivary glands, lungs, thyroid (C cells), bile ducts, and gastrointestinal (GI) tract as well as the ectodermal-derived structures: neuronal structures, Merkel cells of skin, and mesodermal-derived structures, such as kidney and reproductive organs (urogenital ridge).
HISTORICAL PERSPECTIVE
Neuroendocrine tumors (NETs) have captured and sustained the attention of scientists for decades. Extensive research done in this field has greatly expanded the understanding and knowledge of this complex system. A brief history of evolution of the system is provided in the following section. More than a century ago, Heidenham (1870) was the first to describe scattered chromaffin-positive cells using potassium dichromate in the GI tract. Shortly thereafter, Kultschitsky (1897) identified the presence of cells exhibiting different polarity from the mucus-secreting cells with basal position in the mucosa. His discovery and study were credited by naming these cells “Kultschitsky” cells. These cells were named enterochromaffin cells by Caccio (1906). The term carcinoid (Karzinoid Tumoren, carcinoma-like) was coined by Oberndorfer (1907) to describe an ileal tumor of characteristic morphology and biologic behavior. Although much earlier Merling (1838) and Langhans (1867) may have published a description of what is considered a carcinoid, Lubarsch (1888) got the credit for first describing in detail the tumor that was later named “carcinoid tumor.” Huebschmann (1910) was said to be the first one who speculated carcinoid tumor was possibly arising from enterochromaffin cells.
Gosset and Masson (1914) in a study of appendiceal tumors titled “Endocrine Tumors of Appendix” demonstrated these cells as being positive for argentaffin reaction. Hamperl (1938) demonstrated positive argyrophilic reactions in larger population of cells than the argentaffin ones in the GI tract and suggested a possible endocrine role for these cells as well. Later on, he also recognized a bronchial counterpart of intestinal carcinoid.
Feyrter (1938) found similar cells with clear cytoplasm (helle zellen) that were dispersed in the mucosa throughout the GI tract, introducing a concept “diffuse endocrine system.”
Around the same time, Ernst and Berta Scharrer were able to demonstrate secretory granules in neuronal cells in the supraoptic nucleus of fish brain suggesting that the hormone might be produced by such neurons. They called these cells neurosecretory neurons. Subsequently, the term neuroendocrine secretion became popular.
In 1969, Pearse indicated that these specialized cells were capable of uptaking and decarboxylating amine precursors, introducing the concept of Amine Precursors Uptaking and Decarboxylation (APUD) system. He also postulated that APUD cells originated from the neural crest on the basis of the similarities between neuron and neuroendocrine cells. However, this concept was soon refuted by Fontaine and LeDouarin’s (1977) embryonic study employing chick-quail chimera system with interruption of neural crest migration. The study supported
the concept that the neuroendocrine neoplasms derived from stem cells.
the concept that the neuroendocrine neoplasms derived from stem cells.
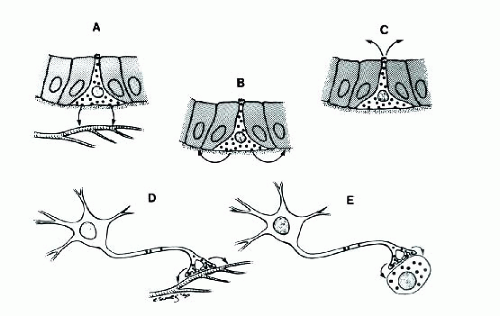
Neuroendocrine-programmed cells are now known to have more complex modes of secretion with paracrine, autocrine, and even exocrine secretion of cell products, in addition to endocrine secretion (Fig. 1.2). Furthermore, some cells are capable of secreting different cytoplasmic products through both exocrine and endocrine secretory mode, referred to as amphicrine. Typical example is illustrated in a goblet cell carcinoid tumor of the appendix (Fig. 1.3)
TAXONOMY OF NEUROENDOCRINE TUMORS
The classification and nomenclature of neoplasms with neuroendocrine differentiation have generated considerable debate and controversy over the years. The term “carcinoid” connoted a benign behavior to some, even though these tumors had the potential to metastasize and behave in a malignant fashion. Neoplasms with similar morphology were designated different terms, none of which reflected the mode of behavior. It was suggested and recommended that all neuroendocrine neoplasms be first grouped into two main phenotypical categories: the neural phenotype (positive immunoreactivity for neurofilaments but negative for cytokeratins) and epithelial phenotypes (positive for cytokeratins but generally negative for neurofilaments).
Secondly it was recommended that all epithelial type neuroendocrine neoplasms be addressed as neuroendocrine carcinomas and subclassified into three categories with assigned grades: I, II, and III (Wick, 2000). Several other schemes were subsequently recommended, based on (1) site of origin, for example, pulmonary neuroendocrine neoplasms, thymic neuroendocrine neoplasms, pancreatic endocrine neoplasms, GI neuroendocrine neoplasms; (2) tumor grade: well, moderately, and poorly differentiated or low grade, intermediate grade, and high grade; (3) invasive characteristics (especially in GI tract and pancreas); (4) secretory products and functional behavior: gastrinoma, somatostatinoma, glucagonoma, etc. The new schemes were two-tiered (NET and neuroendocrine carcinoma for GI tract and pancreas or well-differentiated and poorly differentiated for thymus), three-tiered, or the traditional four-tiered classifications. There remained no consistency. Even with different classification schemes, the term “carcinoid” is entrenched in the medical literature. It is still being
widely used as judged from the most current literature. For this reason, and to simplify the terminologies, the author has decided to retain the term “carcinoid” and also follow the time-honored, four-tiered terminology, especially for pulmonary NETs in this atlas, along with corresponding newer terminologies recommended for any particular site or system. However, for the epithelial phenotypes, the terms such as pituitary adenoma/carcinoma, parathyroid adenoma/carcinoma, thyroid medullary carcinoma, and Merkel cell carcinoma of the skin are retained. The terminologies for the neural phenotypes, for example, pheochromocytoma of adrenal medulla, extra-adrenal paragangliomas, retinoblastoma of eye, Primitive neuroectodermal tumors (PNET)/Ewing sarcoma for soft tissues, and neuroblastoma/medulloblastoma are also retained
widely used as judged from the most current literature. For this reason, and to simplify the terminologies, the author has decided to retain the term “carcinoid” and also follow the time-honored, four-tiered terminology, especially for pulmonary NETs in this atlas, along with corresponding newer terminologies recommended for any particular site or system. However, for the epithelial phenotypes, the terms such as pituitary adenoma/carcinoma, parathyroid adenoma/carcinoma, thyroid medullary carcinoma, and Merkel cell carcinoma of the skin are retained. The terminologies for the neural phenotypes, for example, pheochromocytoma of adrenal medulla, extra-adrenal paragangliomas, retinoblastoma of eye, Primitive neuroectodermal tumors (PNET)/Ewing sarcoma for soft tissues, and neuroblastoma/medulloblastoma are also retained
The neural phenotype includes neuroblastomas and related tumors, PNET/Ewing sarcoma, and neoplasms of the paraganglia (adrenal medulla and extra-adrenal), while epithelial phenotype includes the majority of neuroendocrine cells and their tumors occurring in the viscera. There are often overlapping features between neural and epithelial phenotypes. Examples are the tumors particularly of foregut-derived organs such as those seen in the larynx, lung, thymus, and thyroid, which exhibit spindle cell morphology in some and frequent association with Schwann-like cells (sustentacular cells).
MIXED ADENONEUROENDOCRINE CARCINOMA
These neoplasms consist of both exocrine and neuroendocrine components. At least 30% of tumor volume must be composed of either adenocarcinoma or neuroendocrine neoplasm. Adenocarcinoma with scattered neuroendocrine cells is not considered to be mixed adenoneuroendocrine carcinoma (MANEC).
GENERAL FEATURES OF NEUROENDOCRINE TUMORS AND SHARED CHARACTERISTICS
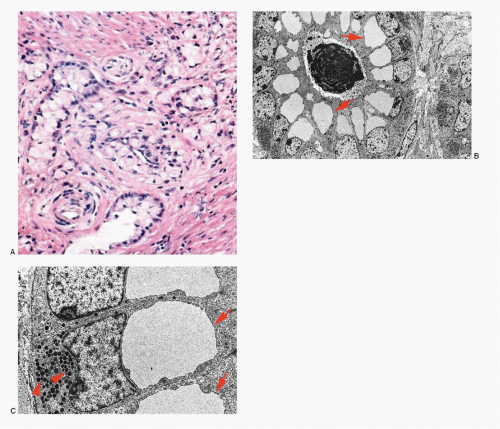
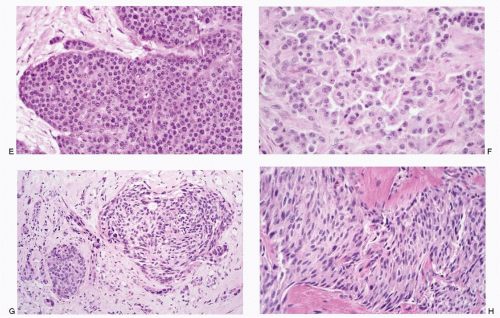
Neuroendocrine-programmed cells present various histomorphologic and cytomorphologic appearances (Figs. 1.4,1.5,1.6,1.7,1.8 and 1.9). The common denominator for neuroendocrine-programmed cells is the presence of small secretory granules, often referred to as neurosecretory granules, crowding the basal or peripheral portion of the cytoplasm. These cells are readily recognized by ultrastructural identification of neurosecretory granules or immunohistochemical expression of “neuroendocrine” markers.
TUMOR GROWTH PATTERN
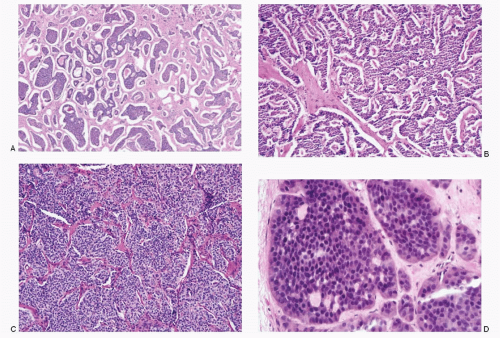
Various growth patterns are observed in neuroendocrine neoplasms (Figs. 1.4,1.5 and 1.6). Well-differentiated NET of epithelial phenotype has so-called organoid architecture either in solid nests (insular pattern or classic type), trabecular, gyriform, or combinations there of. Typical carcinoid tumors, particularly of endodermal-derived organs, display rather unique pattern for each segment of different embryologic derivation: trabecular in foregut, nesting in midgut carcinoid, and mixed in hind gut carcinoid (see Table 4.2).
Tumors with primitive neural phenotype such as seen in neuroblastomas or primitive neuroectodermal tumors exhibit undifferentiated small cells arranged in sheets (see Figs. 16.1 and 17.1). Cells in paraganglioma often form nests, commonly referred to as Zellballen (Fig. 1.6). NETs of neural phenotype are typically associated with Schwannlike cells, designated as sustentacular cells. Schwann-like cells are also frequently seen in some NETs of epithelial phenotype, not only in tissues of foregut derivation but also in tumors with classical neuroendocrine growth pattern.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree