KEY POINTS
Patients with obstructive sleep apnea require evaluation to determine the specific anatomic site(s) of involvement. Long-term cardiovascular problems are a significant concern in these patients.
Repair of traumatic soft tissue injuries requires precise re-alignment of anatomic landmarks such as the grey line and vermilion border.
The key principle in the surgical repair of facial fractures is immobilization, which may require plates, screws, wires, and/or intermaxillary fixation.
Concurrent abuse of tobacco and alcohol are synergistic in increasing the risk of developing head and neck cancer
Monomodality therapy (surgery or radiation) is used for early stage (I/II) head and neck cancer, whereas combination surgery and chemoradiation is utilized with advanced stage (III/IV) malignancies.
Infectious conditions of the head and neck may present with life-threatening sequelae such as loss of airway or intracranial extension.
Disorders of the head and neck can cause significant cosmetic and functional impairment. The practitioner must be empathetic to the effect of these morbidities on quality of life.
Hoarseness, odynophagia, referred otalgia, nonhealing oral ulceration and/or cervical lymphadenopathy present for >2 weeks duration require consideration for subspecialty consultation for evaluation.
A COMPLEX REGION
The head and neck constitute a complex anatomic region where different pathologies may affect an individual’s ability to see, smell, hear, speak, obtain nutrition and hydration, or breathe. The use of a multidisciplinary approach to many of the disorders in this region is essential in an attempt to achieve the best functional results with care. This chapter reviews many of the common diagnoses encountered in the field of otolaryngology–head and neck surgery—and aims to provide an overview that clinicians can use as a foundation for understanding of this region. As is the case with every field of surgery, care for patients with disorders of the head and neck is constantly changing as issues of quality of life and the economics of medicine continue to evolve.
BENIGN CONDITIONS OF THE HEAD AND NECK
Infections may involve the external, middle, and/or internal ear. In each of these scenarios, the infection may follow an acute or chronic course and may be associated with both otologic and intracranial complications. Otitis externa typically refers to infection of the skin of the external auditory canal.1 Acute otitis externa is commonly known as swimmer’s ear, because moisture that persists within the canal after swimming often initiates the process and leads to skin maceration and itching. Typically, the patient subsequently traumatizes the canal skin by scratching (i.e., with a cotton swab or fingernail), thus eroding the normally protective skin/cerumen barrier. Because the environment within the external ear canal is already dark, warm, and humid, it then becomes susceptible to rapid microbial proliferation and tissue cellulitis. The most common organism responsible is Pseudomonas aeruginosa, although other bacteria and fungi may also be implicated. Symptoms and signs of otitis externa include itching during the initial phases and pain with swelling of the canal soft tissues as the infection progresses. Infected, desquamated debris accumulates within the canal. In the chronic inflammatory stage of the infection, the pain subsides, but profound itching occurs for prolonged periods with gradual thickening of the external canal skin. Standard treatment requires removal of debris under otomicroscopy and application of appropriate topical antimicrobials, such as neomycin/polymyxin or quinolone-containing eardrops, which often include topical steroid such as hydrocortisone or dexamethasone to nonspecifically decrease pain and swelling. Nonantibiotic antimicrobial preparations, such as 2% acetic acid, may also have a role, particularly for mixed bacterial/fungal infections. For this reason, the patient should also be instructed to keep the ear dry. Systemic antibiotics are reserved for those with severe infections, diabetics, and immunosuppressed patients.
Diabetic, elderly, and immunodeficient patients are susceptible to a condition called malignant otitis externa, a fulminant necrotizing infection of the otologic soft tissues combined with osteomyelitis of the temporal bone. In addition to the previously mentioned findings, cranial neuropathies may be observed. The classic physical finding is granulation tissue along the floor of the external auditory canal (EAC). Symptoms include persistent otalgia for longer than 1 month and purulent otorrhea for several weeks. These patients require aggressive medical therapy; including IV antibiotics covering Pseudomonas.2 Other gram-negative bacteria and fungi are occasionally implicated, necessitating culture-directed therapy in those cases. Patients who do not respond to medical management require surgical debridement. This condition may progress to involvement of the adjacent skull base and soft tissues, meningitis, brain abscess, and death.
In its acute phase, otitis media typically implies a bacterial infection of the middle ear. This diagnosis accounts for 25% of pediatric antibiotic prescriptions and is the most common bacterial infection of childhood. Most cases occur before 2 years of age and are secondary to immaturity of the Eustachian tube. Contributing factors include upper respiratory viral infection and day-care attendance, as well as craniofacial conditions affecting Eustachian tube function, such as cleft palate. It is also possible that social factors such as day-care attendance and the inappropriate prescribing of antibiotics have led to antibiotic resistance.
Classification of the infection as acute is based upon the duration of the process being less than 3 weeks. In this phase, otalgia and fever are the most common symptoms and physical exam reveals a bulging, opaque tympanic membrane (Fig. 18-1). The most common organisms responsible are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. If the process lasts 3 to 8 weeks, it is deemed subacute. Chronic otitis media, lasting more than 8 weeks, usually results from an unresolved acute otitis media. About 20% of patients demonstrate a persistent middle ear effusion 8 weeks after resolution of the acute phase. Rather than a purely infectious process, however, it represents chronic inflammation and hypersecretion by the middle ear mucosa associated with Eustachiantube dysfunction, viruses, allergy, ciliary dysfunction, and other factors. The bacteriology is variable, but often includes those found in acute otitis media and may be polymicrobial. The exact role of bacteria in the pathophysiology is controversial. The patient experiences otalgia, ear fullness, and conductive hearing loss. Physical examination reveals a retracted tympanic membrane that may exhibit an opaque character or an air-fluid level. Bubbles may be seen behind the retracted membrane.
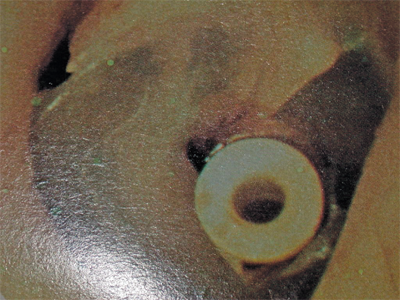
Treatment for uncomplicated otitis media is oral antibiotic therapy. However, penicillin resistance of the commonly implicated organisms is rising such that almost 100% of Moraxella, 50% to 70% of Haemophilus, and up to 40% of pneumococcal strains are resistant.3 Beta-lactamase-resistant combinations, cephalosporins, and macrolides are often required, although amoxicillin and sulfas are still considered first-line drugs. Chronic otitis media is frequently treated with myringotomy and tube placement (Fig. 18-2). This is indicated for frequent acute episodes, chronic effusions persisting beyond 3 months, and those associated with significant conductive hearing loss. The purpose of this procedure is to remove the effusion and provide a route for middle ear ventilation. Tympanic membrane perforation during acute otitis media frequently results in resolution of severe pain and provides for drainage of purulent fluid and middle ear ventilation. These perforations will heal spontaneously after the infection has resolved in the majority of cases. Chronic otitis media, however, may be associated with nonhealing tympanic membrane perforations. Patients may have persistent otorrhea, which is treated with topical drops. Preparations containing aminoglycoside are avoided, because this class of drugs is toxic to the inner ear. Solutions containing alcohol or acetic acid may be irritative or caustic to the middle ear, and are also avoided in the setting of a perforation.
Nonhealing perforation requires surgical closure (tympanoplasty) after medical treatment of any residual acute infection. Chronic inflammation may also be associated with erosion of the ossicular chain, which can be reconstructed with various prostheses or autologous ossicular replacement techniques. Cholesteatoma is an epidermoid cyst of the middle ear and/or mastoid, which causes bone destruction secondary to its expansile nature and through enzymatic destruction. Cholesteatoma develops as a consequence of Eustachian tube dysfunction and chronic otitis media secondary to retraction of squamous elements of the tympanic membrane into the middle ear space. Squamous epithelium may also migrate into the middle ear via a perforation. Chronic mastoiditis that fails medical management or is associated with cholesteatoma is treated by mastoidectomy.
Complications of otitis media may be grouped into two categories: intratemporal (otologic) and intracranial.4 Fortunately, complications are rare in the antibiotic era, but mounting antibiotic resistance necessitates an increased awareness of these conditions. Intratemporal complications include acute coalescent mastoiditis, petrositis, facial nerve paralysis, and labyrinthitis. In acute coalescing mastoiditis, destruction of the bony lamellae by an acute purulent process results in severe pain, fever, and swelling behind the ear. The mastoid air cells coalesce into one common space filled with pus. Mastoid infection may also spread to the petrous apex, causing retro-orbital pain and sixth-nerve palsy. These diagnoses are confirmed by computed tomographic (CT) scan.
Facial nerve paralysis may also occur secondary to an acute inflammatory process in the middle ear or mastoid.5 Intratemporal complications are managed by myringotomy tube placement in addition to appropriate IV antibiotics. In acute coalescent mastoiditis, and petrositis, mastoidectomy is also performed as necessary to drain purulent foci. Labyrinthitis refers to inflammation of the inner ear. Most cases are idiopathic or are secondary to viral infections of the endolymphatic space. The patient experiences vertigo with sensorineural hearing loss and symptoms may smolder over several weeks. Labyrinthitis associated with middle ear infection may be serous or suppurative. In the former case, bacterial products and/or inflammatory mediators transudate into the inner ear via the round window membrane, establishing an inflammatory process therein. Total recovery is eventually possible after the middle ear is adequately treated. Suppurative labyrinthitis, however, is a much more toxic condition in which the acute purulent bacterial infection extends into the inner ear and causes marked destruction of the sensory hair cells and neurons of the eighth-nerve ganglion. This condition may hallmark impending meningitis and must be treated rapidly. The goal of management of inner ear infection, which occurs secondary to middle ear infection, is to “sterilize” the middle ear space with antibiotics and the placement of a myringotomy tube.
Meningitis is the most common intracranial complication. Otologic meningitis in children is most commonly associated with a H. influenzae type B infection. Other intracranial complications include epidural abscess, subdural abscess, brain abscess, otitic hydrocephalus (pseudotumor), and sigmoid sinus thrombophlebitis. In these cases, the otogenic source must be urgently treated with antibiotics and myringotomy tube placement. Mastoidectomy and neurosurgical consultation may be necessary.
Bell’s palsy, or idiopathic facial paralysis, may be considered within the spectrum of otologic disease given the facial nerve’s course through the temporal bone. This entity is the most common etiology of facial nerve paralysis and is clinically distinct from that occurring as a complication of otitis media in that the otologic exam is normal. Historically, Bell’s palsy was synonymous with “idiopathic” facial paralysis. It is now accepted, however, that the majority of these cases represent a viral neuropathy caused by herpes simplex. Treatment includes oral steroids plus antiviral therapy (i.e., valacyclovir). Complete recovery is the norm, but does not occur universally, and selected cases may benefit from surgical decompression of the nerve within its bony canal. Electrophysiologic testing has been used to identify those patients in whom surgery might be indicated.6 The procedure involves decompression of the nerve via exposure in the mastoid and middle cranial fossa. Varicella zoster virus may also cause facial nerve paralysis when the virus reactivates from dormancy in the nerve. This condition, known as Ramsay Hunt syndrome, is characterized by severe otalgia followed by the eruption of vesicles of the external ear. Treatment is similar to Bell’s palsy, but full recovery is only seen in approximately two-thirds of cases.
Traumatic facial nerve injuries may occur secondary to accidental trauma or surgical injury. Iatrogenic facial nerve trauma most often occurs during mastoidectomy.7 When the facial nerve is injured during an operative procedure, it is explored. Injury to >50% of the neural diameter of the facial nerve is addressed either with primary re-anastomosis or reconstructed with the use a nerve graft. Complete recovery of nerve function is uncommon in these cases.
Sinusitis is a clinical diagnosis based on patient signs and symptoms.8 The Task Force on Rhinosinusitis (sponsored by the American Academy of Otolaryngology–Head and Neck Surgery) has established criteria to define “a history consistent with sinusitis.” To establish the diagnosis a patient must exhibit at least two major factors or one major and two minor factors. The classification of sinusitis as acute vs. subacute or chronic is primarily based on the time course over which those criteria have been met. If signs and symptoms are present for at least 7 to 10 days, but for less than 4 weeks, the process is designated acute sinusitis. Subacute sinusitis is present for 4 to 12 weeks and chronic sinusitis is diagnosed when the patient has had signs and symptoms for at least 12 weeks. In addition, the diagnosis of chronic sinusitis requires some objective demonstration of mucosal inflammatory disease. This may be accomplished by endoscopic or radiologic examination (i.e., CT scan).
Acute sinusitis typically follows a viral upper respiratory infection whereby sinonasal mucosal inflammation results in closure of the sinus ostium. This results in stasis of secretions, tissue hypoxia, and ciliary dysfunction. These conditions promote bacterial proliferation and acute inflammation. The mainstay of treatment is the use of antibiotics that are empirically directed toward the three most common organisms S. pneumoniae, H. influenzae, and M. catarrhalis. As with otitis media, antibiotic resistance is a mounting concern. Nosocomial acute sinusitis frequently involves Pseudomonas or S. aureus, both of which may also exhibit significant antibiotic resistance. Other treatments include topical and systemic decongestants, nasal saline spray, topical nasal steroids, and oral steroids in selected cases. In the acute setting, surgery is reserved for complications or pending complications, which may include extension to the eye (orbital cellulitis or abscess) or the intracranial space (meningitis, intracranial abscess). It should also be noted that, strictly speaking, a viral upper respiratory infection (common cold) is a form of acute sinusitis. The working definition outlined previously, however, attempts to exclude these cases by requiring that symptoms be present for at least 7 to 10 days, by which time the common cold should be in a resolution phase. Use of this working definition strives to avoid unnecessary antibiotic prescriptions and further promotion of resistance.
Chronic sinusitis represents a heterogeneous group of patients with multifactorial etiologies contributing to ostial obstruction, ciliary dysfunction, and inflammation. Components of genetic predisposition, allergy, anatomic obstruction, bacteria, fungi, and environmental factors play various roles, depending on the individual patient.8 Diagnosis is suspected with clinical signs and symptoms persisting for at least 12 weeks. Chronic sinusitis may also be associated with the presence of nasal polyps, particularly when there is heavy eosinophilic inflammation. Mucosal inflammation in nonpolypoid chronic sinusitis is predominantly mediated by neutrophils, or is mixed in nature.
Nasal endoscopy is a critical element of the diagnosis of chronic sinusitis. Anatomic abnormalities, such as septal deviation, nasal polyps, and purulence may be observed (Fig. 18-3 and 18-4). The finding of purulence or polypoid change by nasal endoscopy is supportive of the diagnosis of chronic sinusitis, if symptoms persist for at least 12 weeks. In this setting, purulence may represent an acute exacerbation of chronic sinusitis. Pus found on endoscopic exam may be cultured, and subsequent antibiotic therapy can be directed accordingly. Further, the spectrum of bacteria found in chronic sinusitis is highly variable and includes higher prevalence of polymicrobial infections and antibiotic-resistant organisms. Overall, S. aureus, coagulase-negative staphylococci, gram-negative bacilli, and streptococci are isolated, in addition to the typical pathogens of acute sinusitis. Thus, the increased prevalence of community acquired methicillin resistant S. aureus is a mounting concern.9
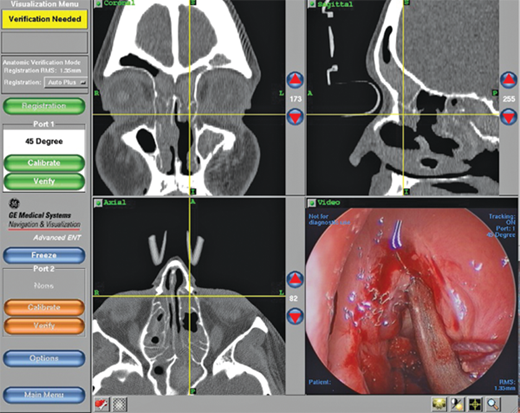
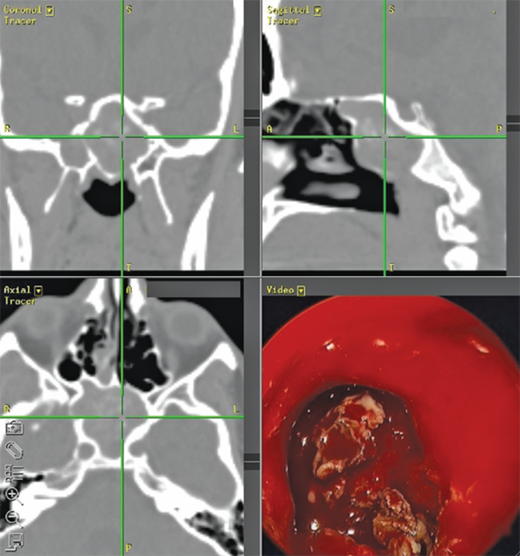
The diagnosis of chronic sinusitis can be confirmed by CT scan, which demonstrates mucosal thickening and/or sinus opacification. It should be underscored, however, that CT scan is not the positive gold standard because many asymptomatic patients will demonstrate findings on a sinus CT scan, and many patients with presumed sinusitis will have negative findings. CT scan has excellent negative predictive value when performed in the setting of active symptoms. Thus, if a patient complains of sinusitis-like symptoms but has no specific physical (endoscopic) findings, and the scan is negative, other diagnoses (e.g., allergies, migraines, tension headaches) should be sought. This has led to the utility of point-of-care CT (POC-CT) scan that can be performed in the physician’s office. POC-CT utilizes cone beam technology10, which acquires the equivalent of >100 axial slices in approximately 1 minute at an effective resolution of 0.3 mm or less. The equipment occupies a room of 8′ × 10′ and can thus be accommodated in almost any office setting (Fig. 18-5). Perhaps most important, the radiation dosing for even the most sophisticated protocol is 0.17 mSv, which is <10% the dose of a conventional head CT and equivalent to approximately 20 days of background radiation. One theoretical shortcoming of this technology is that it does not permit soft tissue imaging. This is seldom a concern in sinonasal evaluation, as this is typically undertaken in bone windows. The acquired data are immediately formatted into triplanar (axial, sagittal, coronal) reconstructions and is also compatible with devices used for intraoperative stereotactic navigation, which can be used to confirm relationships between the disease process, medial orbital wall, and skull base during surgery (Fig. 18-6 and 18-7). Variations of this and other technologies have also been adapted for intraoperative use to ensure completeness of resection and to update anatomic relationships for further intraoperative stereotaxis. Notably, imaging of the temporal bone for evaluation of middle ear structures can be performed via POC-CT as well.
Figure 18-6.
Triplanar imaging revealing proximity to critical structures such as the orbital wall and skull base. This can be used for diagnosis of sinus opacification as well as stereotactic intraoperative navigation, where endoscope view (lower right) can be radiologically correlated with location in the 3 cardinal planes. This case reflects classic allergic fungal sinusitis where the opacified sinuses are filled with heterogeneous whitish material on CT images. Polyps in the ethmoid cavity are seen on the endoscope image.
Medical management of chronic sinusitis includes a prolonged course of oral antibiotics for 3 to 6 weeks, nasal and/or oral steroids, and nasal irrigations with saline or antibiotic solutions.8 Underlying allergic disease may be managed with antihistamines and possible allergy immunotherapy. Although the role of these treatments in resolving chronic sinusitis remains questionable, they may be considered in patients with comorbid allergic rhinitis or as part of empirical management before consideration of surgery. The use of oral steroids may also be selected empirically, particularly in patients with comorbid chronic airway inflammatory diseases such as nasal polyps, allergic rhinitis, or asthma. The decision to use oral steroids must be individualized with consideration of the risks and side effects of these medications. As yet, there is no consensus regarding what constitutes a “maximum” course of medical therapy that should be attempted before consideration of surgery for chronic sinusitis. It should be noted that unless there is suspicion of neoplasm or pending complication of sinusitis, the decision to proceed with surgery is highly individualized. This is because surgery for uncomplicated chronic sinusitis is elective, and patients who “fail” medical management will exhibit significant variability in symptoms, physical signs, and CT findings. More aggressive medical and surgical management may be necessary in patients with comorbid chronic inflammatory disease of the airways such as allergic rhinitis, nasal polyposis, and asthma. Surgery is typically preformed endoscopically where the goals are to remove polyps, enlarge the natural sinus ostia (see Fig. 18-3), and to remove chronically infected bone to promote both ventilation and drainage of the sinus cavities. Inspissated mucin or pus is drained and cultured. Eventual resolution of the chronic inflammatory process can be attained with a combination of meticulous surgery and directed medical therapy, although the patient must understand that surgery may not alter the underlying immunologic pathophysiology. In cases where resection of inflammatory tissue and polyps are not required, recent trends have also included use of angioplasty-type balloons to dilate sinus ostia. The exact role for this technology is unclear, but appears to have promise in outpatient office management of patients with focal or limited obstructive pathology.
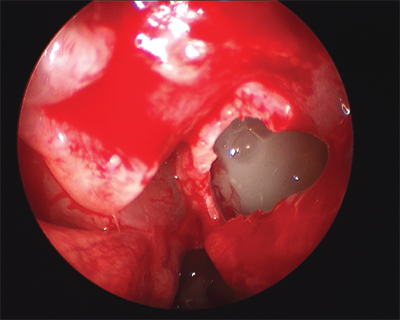
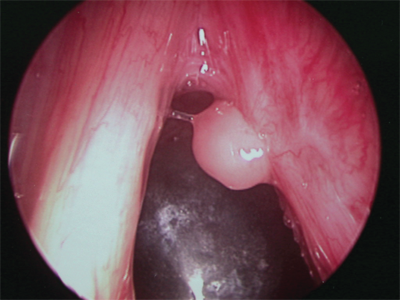
The role of fungi in sinusitis is an area of active investigation. Fungal sinusitis may take on both noninvasive and invasive forms. The noninvasive forms include intracavitary fungal ball and allergic fungal sinusitis, both of which occur in immunocompetent patients. A fungal ball is typically seen in individuals with chronic (or recurrent acute) symptoms that are often subtle and limited to a single sinus. Patients may complain about the perception of a foul odor and occasionally report expelling crusty debris upon nose blowing. Fungus balls represent a significant proportion of isolated sphenoid sinus pathology (Fig. 18-7). The most common scenario, however, is surgery to remove the debris and re-establish sinus ventilation, which is almost always curative.
Classic allergic fungal sinusitis is thought to involve direct stimulation of eosinophils by a subset of helper T cells (TH2) primed by fungal antigens. Patients often present with chronic sinusitis that has been especially refractory to medical management. CT scan has characteristic features, and endoscopic evaluation reveals florid polyposis and inspissated mucin containing fungal debris and products of eosinophil breakdown. The implicated organisms are usually those of the Dematiaceae family, but Aspergillus species are also seen.11 Treatment includes systemic steroids, surgery, and nasal irrigations. Oral antifungal therapy is sometimes indicated as well.
Immunocompetent patients may occasionally develop an indolent form of invasive fungal sinusitis, but more commonly, invasive fungal sinusitis affects immunocompromised patients, diabetics, or the elderly.11 Fungal invasion of the microvasculature causes ischemic necrosis and black eschar of the sinonasal mucosa. Aspergillus and fungi of the Mucoraceae family are often implicated with the latter more common in diabetic patients. Treatment requires aggressive surgical debridement and IV antifungals, but the prognosis is dismal.12
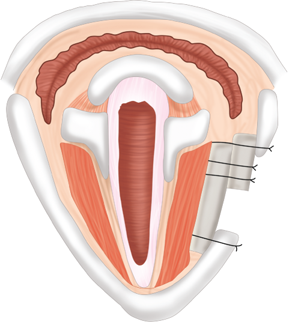
The pharyngeal mucosa contains significant concentrations of lymphoid tissue, predisposing this area to reactive inflammatory changes. Lymphoid tissue of various pharyngeal subsites forms the so-called Waldeyer’s ring, consisting of the palatine tonsils (“the tonsils”), lingual tonsil (lymphoid tissue accumulation within the tongue base), and adenoid. The mucosa of the posterior and lateral pharyngeal walls is also rich with lymphoid cells. Infection, immune-mediated inflammatory disease, or local stressors, such as radiation or acid reflux, may initiate lymphoid reactivity and associated symptoms. Chronic or recurrent adenotonsillitis and adenotonsillar hypertrophy are the most common disorders affecting these structures.
In the vast majority of cases, infectious pharyngitis is viral rather than bacterial in origin. Most cases resolve without complication from supportive care and possibly antibiotics. Patients with tonsillitis typically present with sore throat, dysphagia, and fever. The mucosa is inflamed. Tonsillar exudates and cervical adenitis may be seen, especially when the etiology is bacterial. If adenoiditis is present, symptoms may be similar to those of sinusitis. Objective evaluation of the adenoid requires endoscopy and/or radiographic imaging (lateral neck soft-tissue X-ray). Tonsillitis and adenoiditis may follow acute, recurrent acute, and chronic temporal patterns.
It should be noted, however, that clinical diagnosis often is inaccurate for determining whether the process is bacterially induced. When the patient also has hoarseness, rhinorrhea, cough, and no evidence of exudates or adenitis, an upper respiratory viral infection can be presumed. When a bacterial cause is suspected antibiotics should be initiated to cover the usual organisms: group A beta-hemolytic streptococci (Streptococcus pyogenes), S. pneumoniae, and group C and G streptococci.13 H. influenzae and anaerobes also have been implicated. It is particularly important to identify group A beta-hemolytic streptococci in pediatric patients to initiate timely antibiotic therapy, given the risk of rheumatic fever, which may occur in up to 3% of cases if antibiotics are not used. Historically, if bacterial pharyngitis was suspected in a child, oropharyngeal swab with culture was performed to identify group A beta-hemolytic streptococci. Currently, rapid antigen assays are available with sensitivity and specificity of approximately 85% and 90%, respectively. Some experts advocate culture only when these are negative. Unnecessary antibiotic therapy for patients who are unlikely to have a bacterial etiology should be avoided, given the already mounting antibiotic resistance problem. When suspicion for a bacterial process is high, or with positive culture/antigen assay results, treatment may include penicillin, cephalosporin, or macrolide antibiotics in penicillin-allergic patients.
Complications of S. pyogenes pharyngitis may be systemic, including rheumatic fever, poststreptococcal glomerulonephritis, and scarlet fever. The incidence of glomerulonephritis is not influenced by antibiotic therapy. Scarlet fever results from production of erythrogenic toxins by streptococci. This causes a punctate rash, first appearing on the trunk and then spreading distally, sparing the palms and soles. The so-called strawberry tongue also is seen. Locoregional complications include peritonsillar abscess and, rarely, deep-neck space abscess. Peritonsillar abscess is typically drained with transoral technique under local anesthesia, as is the authors’ practice, but some suggest that needle aspiration without incision is sufficient.13 Deep neck space abscess, which more commonly is odontogenic in origin, usually requires operative incision and drainage via a transcervical approach.
Candida albicans is the most common fungal organism to cause pharyngitis. This organism is a normal component of the oral flora, but under conditions of immunosuppression, broad-spectrum antibacterial therapy, poor oral hygiene, or vitamin deficiency, it may become pathogenic. Whitish-cheesy or creamy mucosal patches are observed with underlying erythema. Diagnosis is easily established by Gram’s stain of this material, revealing budding yeast and pseudohyphae. Oral (-azole) and topical (nystatin) antifungals are usually effective, and immunosuppressed patients may require prophylactic therapy.
Atypical cases of pharyngitis may be caused by Corynebacterium diphtheriae, Bordetella pertussis (whooping cough), Neisseria gonorrhoeae, and secondary syphilis. Diphtheria and pertussis are fortunately rare in developed countries as a result of pediatric vaccination. Atypical viral causes include herpes simplex virus, Epstein-Barr virus (EBV), cytomegalovirus, and HIV are associated with pharyngitis. Systemic EBV infection represents clinical mononucleosis, although syphilis, cytomegalovirus, and HIV are known to cause mononucleosis-like syndromes. These conditions, particularly EBV, may exhibit an exudative pharyngotonsillitis that may be confused with a bacterial etiology. Progression of the clinical picture reveals lymphadenopathy, splenomegaly, and hepatitis. Diagnosis is established based on the detection of heterophile antibodies or atypical lymphocytes in the peripheral blood. Occasionally, pharyngeal biopsy specimen or cervical lymph node biopsy specimen is required to establish the diagnosis.
Noninfectious causes of pharyngitis must also be considered. These include mucositis from chemoradiation therapy, which may be associated with fungal superinfection. Pharyngitis may also be seen in immune-mediated conditions such as erythema multiforme, bullous pemphigoid, and pemphigus vulgaris. In addition, reflux is being increasingly identified as a cause of both laryngitis and pharyngitis, particularly when the symptoms are chronic. A 24-hour pH probe is the gold standard diagnostic test, and treatment is usually successful with lifestyle modification, although proton pump inhibitors are often prescribed.14
Obstructive adenotonsillar hypertrophy may present with nasal obstruction, rhinorrhea, voice changes, dysphagia, and sleep-disordered breathing or OSA depending on the particular foci of lymphoid tissue involved.
Tonsillectomy and adenoidectomy are indicated for chronic or recurrent acute infection and for obstructive hypertrophy.15 The American Academy of Otolaryngology–Head and Neck Surgery Clinical Indicators Compendium suggests tonsillectomy after three or more infections per year despite adequate medical therapy. Tonsillectomy has also been advocated in children who miss 2 or more weeks of school annually secondary to recurrent tonsil infection. Multiple techniques have been described, including electrocautery, sharp dissection, laser, and radiofrequency ablation. There is no consensus as to the best method. In cases of chronic or recurrent infection, surgery is considered only after failure of medical therapy. Patients with recurrent peritonsillar abscess should undergo tonsillectomy when the acute inflammatory changes have resolved. Selected cases, however, require tonsillectomy in the acute setting for the management of severe inflammation, systemic toxicity, or impending airway compromise. Adenoidectomy, in conjunction with myringotomy and tube placement, may be beneficial for children with chronic or recurrent otitis media.16 This is because the adenoid appears to function as a bacterial reservoir that seeds the middle ear via the Eustachian tube. Adenoidectomy is also the first line of surgical management for children with chronic sinusitis. In addition to acting as a bacterial reservoir, an obstructive adenoid impairs mucociliary clearance from the sinonasal tract into the pharynx.
The primary complications of tonsillectomy include perioperative bleeding, airway obstruction, death, and readmission for dehydration secondary to postoperative dysphagia.17 Complications of adenoidectomy also include hemorrhage, as well as nasopharyngeal stenosis. In both procedures, there is risk of velopharyngeal insufficiency. In the latter condition, nasal regurgitation of liquids and hypernasal speech are experienced. Patients with significant airway obstruction secondary to adenotonsillar hypertrophy are also at risk for postobstructive pulmonary edema syndrome, once the obstruction is relieved by adenotonsillectomy. Overall, bleeding is the most prevalent risk and may require a return trip to the operating room for control. With the exception of bleeding, which is observed in 3% to 5% of patients, most of these complications are rare or self-limiting. It deserves special notation that adenotonsillectomy in a child with Down syndrome requires attention to the cervical spine. Patients with this syndrome may exhibit atlantoaxial instability, resulting in cervical spine injury if the neck is extended for the procedure. Baseline radiographs, with appropriate orthopedic or neurosurgical consultation, are indicated preoperatively.
Sleep disorders represent a continuum from simple snoring to upper airway resistance syndrome to obstructive sleep apnea (OSA).18 Upper airway resistance syndrome and OSA are associated with snoring, excessive daytime somnolence, fatigue, and frequent sleep arousals. In OSA, polysomnogram demonstrates at least 10 episodes of apnea or hypopnea per hour of sleep. The average number of apneas and hypopneas per hour can be used to calculate a respiratory disturbance index, which, along with oxygen saturation, can be used to grade the severity of OSA. These episodes occur as a result of collapse of the pharyngeal soft tissues during sleep. In adults, it should be noted that in addition to tonsil size, factors such as tongue size and body mass index (especially >35 kg/m2) are significant predictors of OSA. Other anatomic findings associated with OSA include obese neck, retrognathia, low hyoid bone, and enlarged soft palate. Surgery should be considered after failure of more conservative measures, such as weight loss, elimination of alcohol use, use of oral appliances to open the airway during sleep, and continuous positive airway pressure (CPAP). Recent trends have also included the use of oral appliances to prevent base of tongue retro-prolapsed and subsequent narrowing of the pharyngeal airway during sleep. Oral appliances are useful to avert simple snoring and mild OSA, but their role in more advanced cases is unclear. It is well accepted that CPAP is much more efficacious than an oral appliance, while the latter is associated with improved patient compliance. Those failing conservative therapies may elect a surgical procedure should be tailored to the particular patient’s pattern of obstruction. In children, surgical management typically involves tonsillectomy and/or adenoidectomy, because the disorder is usually caused, at least in part, by hypertrophy or collapse of these structures. In any individual patient, the anatomy must be carefully evaluated to determine whether the site of airway collapse is in the retropalatal region, retrolingual area, or both. In adults, uvulopalatoplasty is frequently performed to alleviate soft-palate collapse and is the most common operation performed for sleep-disordered breathing. The goal of this procedure is to remove redundant tissue from the uvula and soft palate, along with obstructive tonsillar tissue. This can be accomplished with cold steel, laser, and/or cautery. Adults with significant nasal obstruction may benefit from adenoidectomy, septoplasty, reduction in size of the inferior turbinates, and possibly external nasal surgery. Patients with a significant component of retrolingual obstruction may be candidates for tongue base reduction, tongue base advancement, or hyoid suspension. Additionally, a variety of maxillomandibular advancement procedures also have been described to enlarge the anterior-posterior dimension of the retrolingual airway. Patients with moderate to severe sleep apnea frequently manifest involvement of the tongue base. However, management of this subgroup may be difficult, as procedures addressing the retrolingual airway can involve difficult recovery, significant morbidity, and limited success. These patients often continue to require continuous positive airway pressure despite performance of multilevel surgical procedures. Patients with severe OSA (respiratory disturbance index >40, lowest nocturnal oxygen saturation <70%) and unfavorable anatomy or comorbid cardiopulmonary disease may require tracheotomy,19 which is the only surgical “cure” for OSA. Tracheotomy should be offered in patients with evidence of right heart failure (cor pulmonale), which is a potential sequela of severe OSA or undertreated cases of moderate OSA.
On the opposite end of the spectrum, many patients present with snoring but fail to exhibit OSA according to polysomnographic criteria. These “social snorers” may pursue elective procedures that stiffen the uvula and soft palate. This may be accomplished by the application of radiofrequency energy or cautery to induce submucosal scar, or by palatal implants. Elective uvulopharyngoplasty may also be a consideration in this population.
Disorders of voice may affect a wide array of patients with respect to age, gender, and socioeconomic status. The principal symptom of these disorders, at least when a mass lesion is present, is hoarseness. Other vocal manifestations include hypophonia or aphonia, breathiness, and pitch breaks. Benign laryngeal disorders may also be associated with airway obstruction, dysphagia, and reflux.20 Smoking may also be a risk factor for benign disease, but this element of the history should raise the index of suspicion for malignancy.
Recurrent respiratory papillomatosis (RRP) reflects involvement of human papillomavirus (HPV) within the mucosal epithelium of the upper aerodigestive tract. The larynx is the most frequently involved site, and subtypes 6 and 11 are the most often implicated. The disorder typically presents in early childhood, secondary to viral acquisition during vaginal delivery. Many cases resolve after puberty, but the disorder may progress into adulthood. Adult-onset RRP typically occurs in the third or fourth decade of life, is usually less severe, and is more likely to involve extralaryngeal sites of the upper aerodigestive tract. With laryngeal involvement, RRP is most likely to present with hoarseness, although airway compromise may be observed. The diagnosis can be established with office endoscopy. Currently, there is no “cure” for RRP. Treatment involves operative microlaryngoscopy with excision or laser ablation, and the natural history is eventual recurrence. Therefore, surgery has an ongoing role for palliation of the disease. Multiple procedures are typically required over the patient’s lifetime. Several medical therapies, including intralesional cidofovir injection and oral indole-3-carbinol, are being investigated to determine their abilities to retard recurrence. Additionally, the advent of HPV vaccines has suggested a role for this therapy in prevention of RRP.21
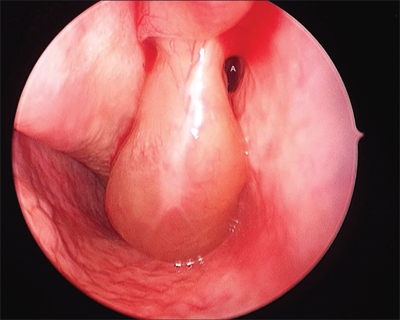
Laryngeal granulomas typically occur in the posterior larynx on the arytenoid mucosa (Fig. 18-8). These lesions are typically secondary to multiple factors,22 including reflux, voice abuse, chronic throat clearing, endotracheal intubation, and vocal fold paralysis. Effective management requires identification of the underlying cause(s). Patients report pain (often with swallowing) more commonly than vocal changes. In addition to fiber-optic laryngoscopy, work-up may include voice analysis, laryngeal electromyography (EMG), and pH probe testing.23 Treatment is individualized, depending on the contributing factors identified. First-line modalities that may be used include voice rest, voice retraining therapy, and anti-reflux therapy. The management of vocal cord paresis/paralysis is discussed later in this section. It is notable that the majority of cases demonstrate a component of reflux and when maximal medical therapy has failed, fundoplication may be indicated. The role of surgical excision is somewhat controversial, because it does not address the underlying etiology and is frequently associated with recurrence. Nonetheless, excision is indicated when carcinoma is suspected or when the patient has airway obstruction. Surgery may also be indicated in selected cases when a granuloma has matured into a fibroepithelial polyp, or when the patient (e.g., a performing artist) requires prompt removal for voice restoration. Surgical excision is optimally performed under jet ventilation so as to avoid endotracheal intubation. During surgery, it is important to preserve the arytenoid perichondrium to promote epithelialization postoperatively.
Edema in the superficial lamina propria of the vocal cord is known as polypoid corditis, polypoid laryngitis, polypoid degeneration of the vocal cord, or Reinke’s edema. The superficial lamina propria just underlies the vibratory epithelial surface. Edema is thought to arise from injury to the capillaries that exist in this layer, with subsequent extravasation of fluid. Patients report progressive development of a rough, low-pitched voice. Females more commonly present for medical attention because the lowered vocal frequency is more evident, given the higher fundamental frequency of the female voice. The etiology is also multifactorial and may involve smoking, laryngopharyngeal reflux, hypothyroidism, and vocal hyperfunction. Most of these patients are heavy smokers and findings are typically bilateral.24
Focal, unilateral hemorrhagic vocal cord polyps are more common in men. These occur secondary to capillary rupture within the mucosa by shearing forces during voice abuse. Use of anticoagulant or antiplatelet drugs may be a risk factor. As with laryngeal granulomas, treatment of polypoid corditis and vocal cord polyps requires addressing the underlying factors. Conservative management includes absolute discontinuance of smoking, reflux management, and voice therapy. Notably, topical and systemic steroids are ineffective for these conditions. For polypoid corditis, elective surgery may be performed under microlaryngoscopy to evacuate the gelatinous matrix within the superficial lamina propria and trim excess mucosa. Focal polyps may be excised superficially under microlaryngoscopy. Surgery, particularly for polypoid corditis, will be less effective in patients who continue to smoke, although it should be noted that because of their heavy smoking history, surgery might be necessary to rule out occult malignancy. Surgery for polypoid corditis and hemorrhagic polyps may be accomplished either with cold steel or by using the carbon dioxide (CO2) laser. Postoperative voice therapy is usually indicated.
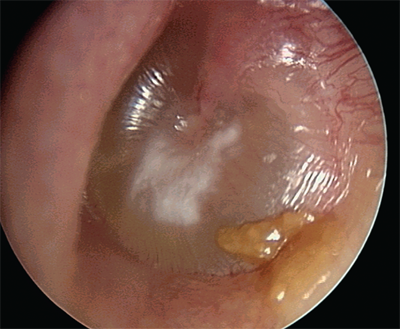
Vocal cord cysts may occur under the laryngeal mucosa, particularly in regions containing mucous-secreting glands, such as the supraglottic larynx. Occasionally, they derive from minor salivary glands, and congenital cysts may persist as remnants of the branchial arch. Cysts may present in a variety of ways depending on the size and site of origin (Fig. 18-9). Cysts of the vocal cord may be difficult to distinguish from vocal polyps, and video stroboscopic laryngoscopy may be necessary to help establish the diagnosis. Cysts observed in children can be quite large, thus compromising the airway. Lesions of the true vocal cord usually present with hoarseness. Treatment again depends on the size and site of the cyst. Large cysts of the supraglottic larynx are treated by marsupialization with cold steel or a CO2 laser. Those of the vocal cord itself require careful microsurgical technique for complete removal of the cyst while preserving the overlying mucosa.
Leukoplakia of the vocal fold represents a white patch (which cannot be wiped off) on the mucosal surface, usually on the superior surface of the true vocal cord. Rather than a diagnosis per se, the term leukoplakia describes a finding on laryngoscopic examination. The significance of this finding is that it may represent squamous hyperplasia, dysplasia, and/or carcinoma. Lesions exhibiting hyperplasia have a 1% to 3% risk of progression to malignancy. In contrast, that risk is 10% to 30% for those demonstrating dysplasia. Furthermore, leukoplakia may be observed in association with inflammatory and reactive pathologies, including polyps, nodules, cysts, granulomas, and papillomas. The wide, differential diagnosis for leukoplakia necessitates sound clinical judgment when selecting lesions that require operative direct laryngoscopy with biopsy specimen for histopathologic analysis. Features of ulceration and erythroplasia are particularly suggestive of possible malignancy. A history of smoking and alcohol abuse should also prompt a malignancy work-up. In the absence of suspected malignancy, conservative measures are used for 1 month. These include reduction of caffeine and alcohol, which are dehydrating and promote laryngopharyngeal reflux, proper hydration, and elimination of vocal abuse behaviors. Antireflux therapy, including proton pump inhibitors, may be prescribed. Investigational therapies, including retinoids, also have been attempted. Any lesions that progress, persist, or recur should be considered for excisional biopsy specimen.
Unilateral vocal cord paralysis is typically iatrogenic in origin,25 following surgery to the thyroid, parathyroid, carotid, or cardiothoracic structures. Vocal cord paralysis may also be secondary to malignant processes in the lungs, thoracic cavity, skull base, or neck. In the pediatric population up to one fourth of cases may be neurologic in origin, with Arnold-Chiari malformation being the most common. Overall, the left vocal cord is more commonly involved secondary to the longer course of the recurrent laryngeal nerve (RLN) on that side, which extends into the thoracic cavity. When anterior approaches to the cervical spine are performed, however, the right RLN is at an increased risk, because it courses more laterally to the tracheoesophageal complex. Neurotoxic medications, trauma, intubation injury, and atypical infections are less common causes of vocal cord paralysis. The cause remains idiopathic in up to 20% of adults and 35% of children. These cases should prompt an imaging work-up to examine the course of the vagal/RLN in question: from the skull base to the aortic arch on the left, and from skull base to the subclavian on the right.
“Idiopathic” left true vocal cord paralysis may be a presenting sign of malignancy involving the lung, thyroid, or esophagus. Adults typically present with hoarseness and the voice may be breathy if the contralateral vocal cord has not compensated to close the glottic valve. If the proximal vagus nerve or the superior laryngeal nerve is involved, the patient may demonstrate aspiration secondary to diminished supraglottic sensation. Stridor, weak cry, and respiratory distress are seen in children, but adults typically do not exhibit signs of airway compromise unless paralysis is bilateral. Flexible fiber-optic laryngoscopy usually confirms the diagnosis, but laryngeal EMG may be necessary to distinguish vocal cord paralysis from mechanical fixation secondary to scar tissue or cricoarytenoid joint fixation. The position of the paralyzed fold depends on the residual innervation, pattern of reinnervation, and the degrees of atrophy and fibrosis of the laryngeal musculature. In bilateral vocal cord paralysis, the cords are often paralyzed in a paramedian position, creating airway compromise that necessitates tracheotomy. Once an airway is secure, vocal cord lateralization or arytenoidectomy may be performed electively to provide an adequate airway. Treatment of unilateral vocal cord paralysis includes speech therapy, which promotes glottic closure to optimize the voice and prevent aspiration. Some patients do well with this modality alone.
Surgical treatment to augment or medialize the paralyzed vocal fold is performed to provide a surface against which the contralateral normal fold may make contact. Injection laryngoplasty may be performed under office or operative laryngoscopy with a variety of autologous (fat, collagen) or alloplastic (hydroxyapatite, hyaluronic acid, micronized cadaveric human collagen) compounds. Teflon injection is of historical significance only secondary to the incidence of severe foreign body inflammatory reactions. Injection of the vocal fold increases its bulk to optimize closure with the contralateral normal fold. This technique also is useful for vocal cord atrophy, which may occur with aging. Laryngeal framework surgery involves the implantation of cartilage, hydroxyapatite, Gore-Tex, or silicone under the musculomembranous fold via an external approach through a window in the thyroid cartilage (Fig. 18-10).26 This may be combined with procedures to adduct the vocal process of the arytenoids. Laryngeal reinnervation (with the ansa cervicalis to the RLN transfer) and pacing have also been attempted with various success.
Vascular lesions can be broadly classified into two groups: hemangiomas and vascular malformations.27 Hemangiomas are the most common vascular lesions present in infancy and childhood. These lesions are present at birth in up to 30% of cases, but usually become apparent in the first few weeks of life. The lesions proliferate in size over the first year before beginning involution, which subsequently occurs over the next 2 to 12 years. While 40% of cases will resolve completely, the remainder will require intervention. Once the proliferative phase has ended, the lesion should be observed every 3 months for involution, and surgery should be considered for those that have not significantly involuted by 3 to 4 years of age. Surgical treatment of proliferating hemangiomas is reserved for lesions associated with severe functional or cosmetic problems, such as those involving the nasal tip or periorbital region. Treatment is performed with either the flashlamp-pumped pulsed-dye laser (FPDL), the potassium titanyl phosphate (KTP) laser, or the neodymium yttrium-aluminum garnet (Nd:YAG) laser, repeated every 4 to 6 weeks until the lesion disappears. Systemic steroids may be used to halt rapidly proliferating lesions until the child reaches 12 to 18 months, after which growth should stabilize or involution begin. Subcutaneous interferon-α-2a may also be used for this purpose. This treatment, however, is associated with neurologic side effects and should be used with caution.27,28 There have also been recent trends of using propranolol to inhibit hemangioma growth, and this practice is gaining popularity in complicated cases.
Vascular malformations, in contrast, are almost always present at birth and slowly enlarge without proliferation.29 These may arise from capillaries, venules, veins, arteriovenous channels, and/or lymphatics. Capillary malformations usually involve the midline neck or forehead, and may fade with age. Venular malformations are also known as port-wine stains. These lesions often follow facial dermatomes and usually thicken with age. Venous malformations are composed of ectatic veins within the lips, tongue, or buccal area. These may present as purple masses or subcutaneous/submucosal nodules. Arteriovenous malformations are rare malformations of arteriovenous channels that failed to regress during development.
Lymphatic malformations or lymphangiomas of the head and neck usually involve the cervical area, in which case they are more commonly macrocystic and well demarcated. Those arising above the hyoid bone tend to be microcystic and have an infiltrative quality. Lymphangiomas may become secondarily infected and may rapidly enlarge, causing airway compromise. These lesions may also be associated with feeding difficulties and failure to thrive.
Capillary hemangiomas and superficial port-wine stains are effectively treated by FPDL. The KTP or Nd:YAG laser is used for deeper port-wine stains. Venous malformations may be treated with laser, sclerotherapy, and/or surgical excision, depending on the depth, size, and location. Superficial lesions are treated with the Nd:YAG laser, which has deeper penetration than either the FPDL or KTP laser. Deeper venous malformations may benefit from Nd:YAG therapy of the superficial component followed by meticulous surgical excision of the deeper component. Sclerotherapy should be undertaken with extreme caution in the head and neck, because the valveless quality of the veins in this region introduces significant risk of cavernous sinus thrombosis. Arteriovenous malformations require formal surgical resection with negative margins. Preoperative angiographic embolization is frequently used to facilitate surgery. Microvascular reconstruction may be necessary, depending on the extent of the resection required. Surgical excision is also required for lymphatic malformations, although superficial lesions are sometimes treatable with the CO2 laser. This often is difficult for microcystic cases given the infiltrative nature. Sclerotherapy with OK-432 is effective in macrocystic lymphangiomas, and multiple other sclerosing agents, including bleomycin, have been explored.30
TRAUMA OF THE HEAD AND NECK
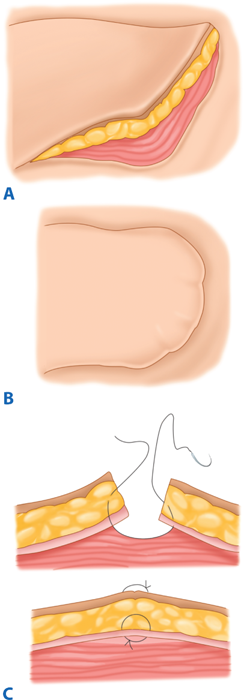
Management of head and neck soft-tissue trauma follows general surgical principles, with several salient features. In the acute setting, patients should be managed with head-of-bed elevation to decrease tissue edema. Most lacerations without significant tissue loss can be closed primarily, which is preferred where possible. Head and neck soft tissues have the benefit of a robust blood supply. Thus, nearly devitalized soft tissues often survive, such that any tissue debridement should be very conservative. Closure of trapdoor lacerations requires conservative undermining of surrounding tissue and good approximation of subdermal levels prior to epidermal closure. A pressure dressing is also applied. These measures are employed to avoid a pincushion deformity (Fig. 18-11). Typically, when repairing facial lacerations, subdermal layers are approximated with an absorbable 3-0 or 4-0 suture such as, Vicryl or polydioxanone, and the skin is closed using 5-0 or 6-0 monofilament nylon or Prolene. Sutures are removed after 4 to 5 days, but may be removed earlier in thin-skinned areas. Systemic antibiotics are reserved for through-and-through mucosal lacerations, contaminated wounds, bite injuries, and when delayed closure is performed (>72 hours). The chosen antibiotic should cover S. aureus. After skin injuries, the patient is instructed to avoid sunlight, because this can cause pigmentary abnormalities in the abrasion or scar line, which matures over a 6- to 12-month period.
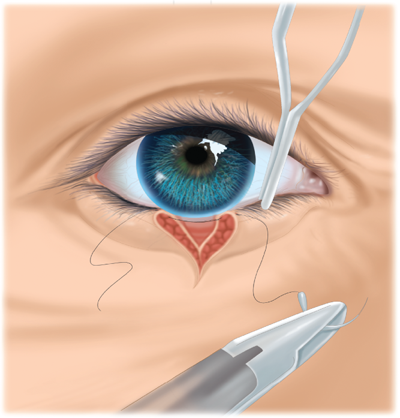
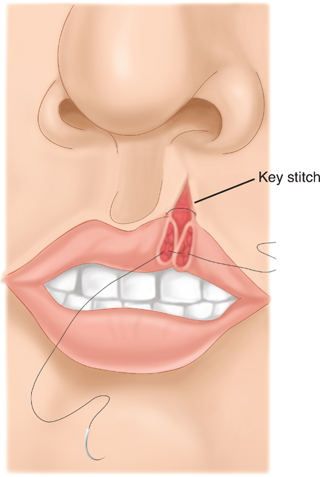
Wound closure must be understood in the context of the cosmetic and functional anatomic landmarks of the head and neck. Management of injuries to the eyelid requires identification of the orbicularis oculi, which is closed in a separate layer. The gray line (conjunctival margin; Fig. 18-12) must be carefully approximated to avoid lid notching or height mismatch. Management of lip injuries follows the same principle. The orbicularis oris must be closed, and the vermilion border carefully approximated (Fig. 18-13). Injuries involving one-fourth the width of the eyelid or one third the width of the lip may be closed primarily; otherwise, flap or grafting procedures may be required. With laceration of the auricle, key structures such as the helical rim and antihelix must be carefully aligned. These injuries must be repaired so that the cartilage is covered. The principles of auricular repair are predicated on the fact that the cartilage has no intrinsic blood supply and is thus susceptible to ischemic necrosis following trauma. The suture should be passed through the perichondrium, while placement through the cartilage itself should be avoided.
Auricular hematomas should be drained promptly, with placement of a bolster as a pressure dressing. A pressure dressing is frequently advocated after closure of an ear laceration. It also deserves note that the surgeon must avoid the temptation to perform aggressive debridement after injuries to the eyelid or auricle. Given the rich vascular supply to the face and neck, many soft-tissue components that appear devitalized will indeed survive.
Most traumatic facial nerve injuries are secondary to temporal bone trauma, which is discussed later in this section. Soft-tissue injuries occurring in the midface may involve distal facial nerve branches. Those injured anterior to a vertical line dropped from the lateral cantus do not require repair secondary to collateral innervation in the anterior midface. Posterior to this line, the nerve should be repaired, primarily if possible, using 8-0 to 10-0 monofilament suture to approximate the epineurium under microscopic visualization. If neural segments are missing, cable grafting is performed using either the greater auricular (provides 7 to 8 cm) or sural nerve (up to 30 cm) as a donor. Injuries to the buccal branch should alert the examiner to a possible parotid duct injury. This structure lies along an imaginary line drawn from the tragus to the midline upper lip, running along with the buccal branch of the facial nerve. The duct should be repaired over a 22-gauge stent or marsupialized into the oral cavity.
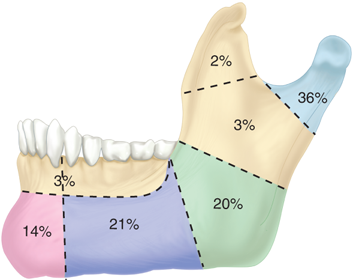
Facial bone fractures most commonly involve the mandible. Fractures most often involve the angle, body, or condyle, and in most cases, two or more sites are almost always involved (Fig. 18-14). Fractures are described as either favorable or unfavorable, depending on whether or not the masticatory musculature tends to pull the fracture into reduction or distraction. Vertically favorable fractures are brought into reduction by the masseter, while horizontally favorable fractures are brought into reduction by the pterygoid musculature. The fracture is usually evaluated by radiographic exam using a Panorex, but specialized plain film views, and occasionally CT scan, are necessary in selected cases. Classical management of mandiblefractures dictated closed reduction and a 4- to 6-week period of intermaxillary fixation (IMF) with arch bars applied via circumdental wiring. Comminuted, displaced, or unfavorable fractures underwent open reduction and wire fixation in addition to IMF. Currently, arch bars and IMF are performed to establish occlusion. The fracture is then exposed and reduced, using transoral approaches where possible.
Transcervical approaches are required to address fractures of the ramus or posterior body, with careful attention given to preserving the marginal mandibular branch of the facial nerve. Rigid fixation is then accomplished by the application of plates and screws. Selected fractures, such as those of the body, benefit from dynamic compression plating, which applies pressure toward the fracture line. With rigid fixation, IMF is required to establish occlusion, and may not be necessary for a full 6 postoperative weeks. This is preferable because IMF is associated with gingival and dental disease, as well as with significant weight loss and malnutrition, during the fixation period. New techniques have included the 4-point fixation technique, where the maxilla and mandible are held in occlusion by wires attached to intraoral cortical bone screws, with two screws above and below the occlusal line anteriorly. In edentulous patients, determining the baseline occlusion is of less significance because dentures may be refashioned once healing is complete. If IMF is required to aid in immobilization of the fracture in an edentulous patient, interosseous wiring and/or the fabrication of custom-made splints is required.
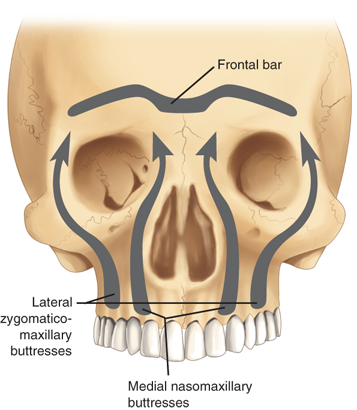
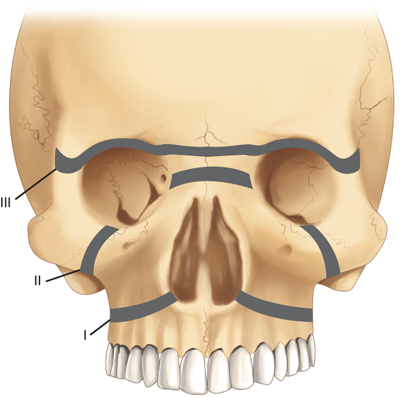
Midface fractures are classically described in three patterns: Le Fort I, II, and III. A full understanding of midface structure is first necessary (Fig. 18-15). Three vertical buttresses support the midface: the nasofrontal-maxillary, the frontozygomaticomaxillary, and pterygomaxillary.31 The five horizontal buttresses include the frontal bone, nasal bones, upper alveolus, zygomatic arches, and the infraorbital region. Classical signs of midface fractures in general include subconjunctival hemorrhage; malocclusion; midface numbness or hypesthesia (maxillary division of the trigeminal nerve); facial ecchymoses/hematoma; ocular signs/symptoms; and mobility of the maxillary complex.
Le Fort I fractures occur transversely across the alveolus, above the level of the teeth apices. In a pure Le Fort I fracture, the palatal vault is mobile while the nasal pyramid and orbital rims are stable. The Le Fort II fracture extends through the nasofrontal buttress, medial wall of the orbit, across the infraorbital rim, and through the zygomaticomaxillary articulation. The nasal dorsum, palate, and medial part of the infraorbital rim are mobile. The Le Fort III fracture is also known as craniofacial disjunction. The frontozygomaticomaxillary, frontomaxillary, and frontonasal suture lines are disrupted. The entire face is mobile from the cranium. It is convenient to conceptualize complex midface fractures according to these patterns (Fig. 18-16); however, in reality, fractures reflect a combination of these three types. Also, the fracture pattern may vary between the left and right sides of the midface. Lateral blows to the cheek may be associated with isolated zygoma fractures. The zygoma is typically displaced inferiorly and medially with disruption of the suture lines between the temporal, frontal, and maxillary bones and the zygoma. Disruption of the latter articulation may be associated with depression into the maxillary sinus and blood in the sinus cavity. Fractures of the midface and/or zygoma may be associated with an orbital blowout, whereas the orbital floor is disrupted and orbital soft tissues subsequently herniate into the maxillary sinus (Fig. 18-17). The mechanism of orbital blowout may involve propagation of adjacent fracture lines or may be the result of a sudden increase in intraorbital pressure during the injury. This may be associated with enophthalmos or entrapment of the inferior oblique muscle. The latter results in diplopia upon upward gaze. Entrapment is confirmed by forced duction testing, where, under topical or general anesthesia, the muscular attachment of the inferior oblique is grasped with forceps and manipulated to determine passive ocular mobility. Fractures of the midface, zygoma, and orbital floor are best evaluated using CT scan, and repair requires a combination of transoral and external approaches to achieve at least two points of fixation for each fractured segment.32 Significant areas of bone loss can be reconstructed with commercially available hydroxyapatite bone cements, an osteoconductive calcium-phosphate matrix. Blowout fractures demonstrating significant entrapment or enophthalmos are treated by orbital exploration and reinforcement of the floor with mesh or bone grafting.
Temporal bone fractures occur in approximately one fifth of skull fractures. As with fractures of the mandible and midface, blunt trauma (from motor vehicle accident or assault) usually is implicated. Unfortunately, the incidence of temporal bone fracture from gunshot wounds to the head is rising. Fractures are divided into two patterns (Fig. 18-18), longitudinal and transverse, based on the clinical picture and CT imaging. In practice, most fractures are oblique. By classical descriptions, longitudinal fractures constitute 80% and are associated with lateral skull trauma. Signs and symptoms include conductive hearing loss, ossicular injury, bloody otorrhea, and labyrinthine concussion. The facial nerve is injured in approximately 20% of cases. In contrast, the transverse pattern constitute only 20% of temporal bone fractures and occurs secondary to fronto-occipital trauma. The facial nerve is injured in 50% of cases. These injuries frequently involve the otic capsule to cause sensorineural hearing loss and loss of vestibular function. Hemotympanum may be observed. A cerebrospinal fluid (CSF) leak must be suspected in temporal bone trauma. This resolves with conservative measures in most cases. The most significant consideration in the management of temporal bone injuries is the status of the facial nerve. Delayed or partial paralysis will almost always resolve with conservative management. However, immediate paralysis that does not recover within 1 week should be considered for nerve decompression. Electroneurography and EMG have been used to help determine which patients with delayed-onset complete paralysis will benefit from surgical decompression. The finding of >90% degeneration more than 72 hours after the onset of complete paralysis is considered an indication for surgery.33 Multiple approaches have been described for facial nerve decompression, some of which require the sacrifice of hearing. These patients may have severe intracranial or vascular injuries such that the decision to operate must also be made in the context of the patient’s overall medical stability. It is of paramount importance to protect the eye in patients with facial nerve paralysis of any etiology, because absence of an intact blink reflex will predispose to corneal drying and abrasion. This requires the placement of artificial tears throughout the day with lubricant ointment, eye taping, and/or a humidity chamber at night.34,35
TUMORS OF THE HEAD AND NECK
When a discussion of neoplasms of the head and neck is initiated, the conversation frequently focuses on squamous cell carcinoma. This is because the majority of malignancies of this region are represented by this pathology. The diagnosis and treatment of lesions spanning from the lips and oral cavity to the larynx and hypopharynx requires a similar methodic approach.
The selection of treatment protocols varies for each site within the upper aerodigestive tract. The importance of multidisciplinary management cannot be underestimated. Presentation of cases before a tumor board allowing review of a patient’s history, physical examination findings, imaging, and prior pathology specimens allows for confirmation of the patient’s status. Additionally, it should encourage discussion from multiple points of view concerning the most appropriate treatment options available. Participation in the discussion with representatives of radiation oncology, medical oncology, surgical oncology, oral maxillofacial surgery/dental medicine, along with radiologists and pathologists specializing in upper aerodigestive tract disorders benefits not only the patient but also represents an excellent teaching opportunity for all disciplines.
The development of organ preservation protocol and the evolution of free tissue reconstructive techniques are some of the most significant advances made within the field during the last two decades. The future of the treatment of head and neck cancer lies within the field of molecular biology. As more is understood about the genetics of cancer, tailoring treatment options to a particular tumor mutation has the capacity to maximize survival while achieving the highest quality of life.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree