Learning Objectives
Anemia
Anemia refers to a deficiency in red blood cells (RBCs) and implies a decline in oxygen-carrying capacity. The complete blood count (CBC) provides several measures of red cell quantity, including RBC count, hemoglobin (Hb) concentration, and hematocrit (Hct) (see description of RBC indices later in this chapter). Hb concentration is the parameter most widely used to diagnose anemia, based on 1967 World Health Organization (WHO) recommendations (Table 10–1). This definition is not universally accepted, and numerous alternatives have been proposed over the years, usually suggesting slightly higher values and race-specific values. It is important to remember also that the normal ranges for Hb and Hct are different for infants, children, adult men, adult women, pregnant women, and the elderly. Attention to age- and gender-appropriate normal ranges is important in the evaluation of anemia.
Anemia refers to a deficiency in red blood cells (RBCs) and implies a decline in oxygen-carrying capacity.
Anemia may present with pallor, fatigue, dyspnea, or evidence of poor tissue oxygenation (chest pain due to poor cardiac oxygenation, altered mental status due to poor cerebral oxygenation). Often, particularly when anemia is mild or the patient is otherwise healthy, anemia presents simply as an abnormal CBC.
Anemia stimulates several compensatory mechanisms. The cardiopulmonary system compensates by attempting to make the most of the blood it has by exchanging more gases (tachypnea), and circulating more volume (tachycardia). The marrow responds with increased erythropoiesis, stimulated by an increase in renal production of erythropoietin (EPO) in response to hypoxia. If the means to create mature red cells are intact (ie, if the underlying cause of the anemia is not a production or maturation defect), then this response can usually succeed. In addition to making more erythrocytes, the marrow begins to release immature erythrocytes into the circulation. Many of these still contain a network of ribosomes and rough endoplasmic reticulum involved in the making of Hb, which identifies them morphologically as reticulocytes (see description of reticulocyte counting later in this chapter). Over the next 3 to 4 days, this endoplasmic reticulum dissolves and a mature RBC results. In very brisk marrow responses, some red cells may be released that still contain a nucleus.
Identifying the cause of anemia is usually fairly straightforward. Examination of the peripheral smear is especially important, since numerous clues can be found there.
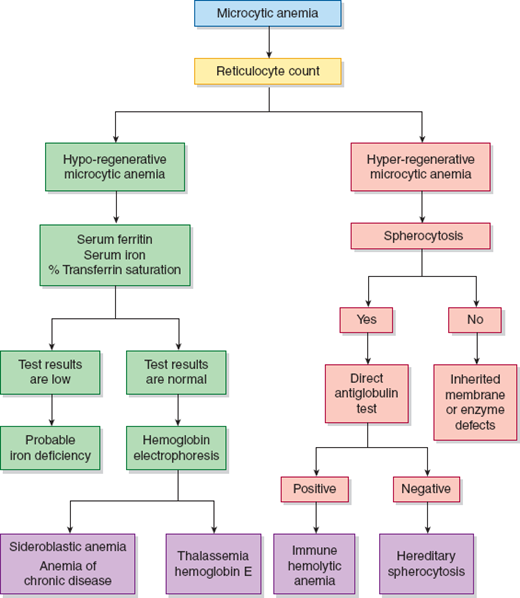
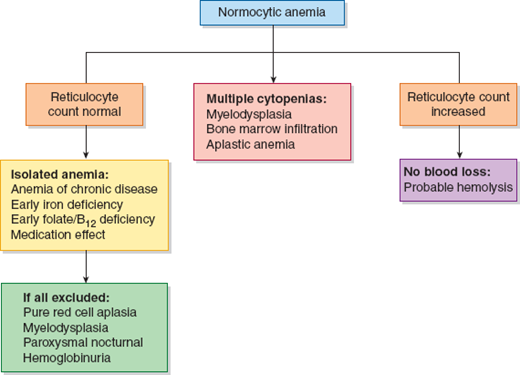
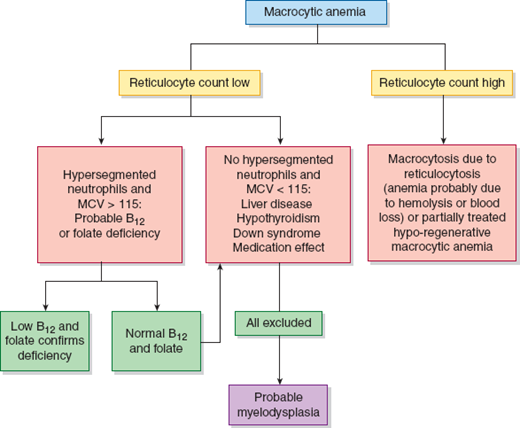
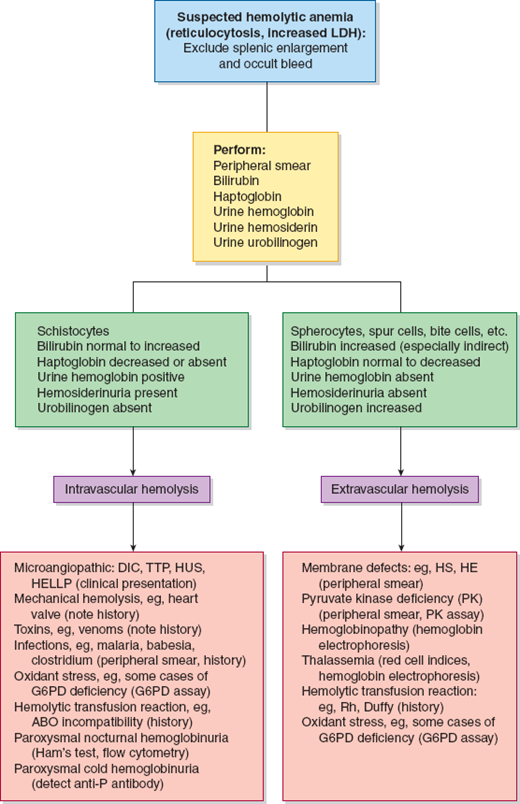
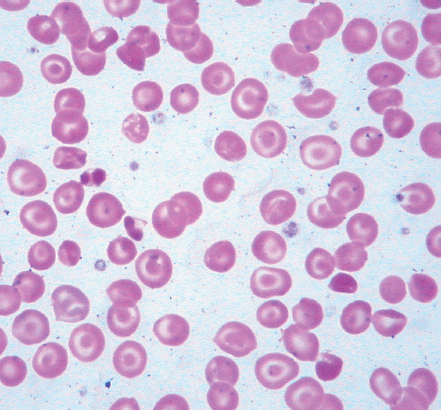
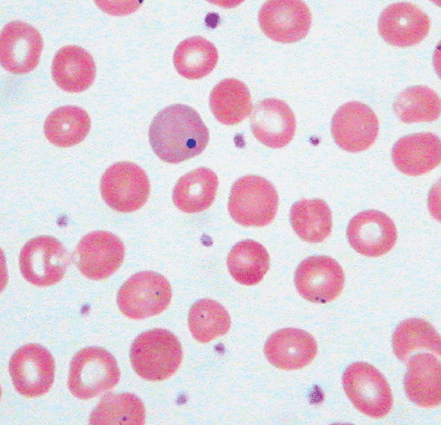
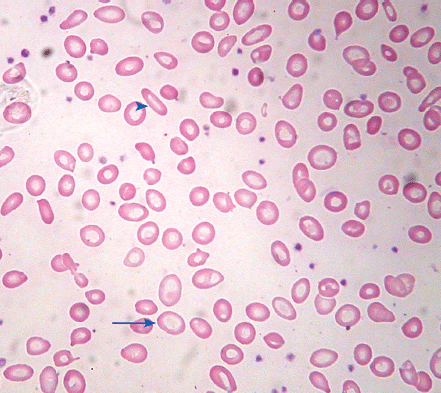
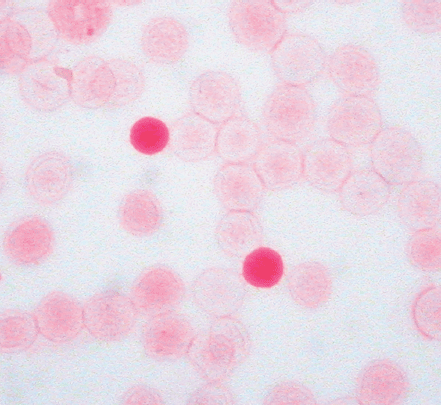
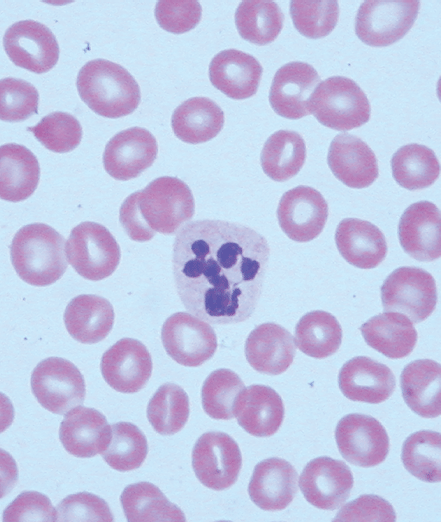
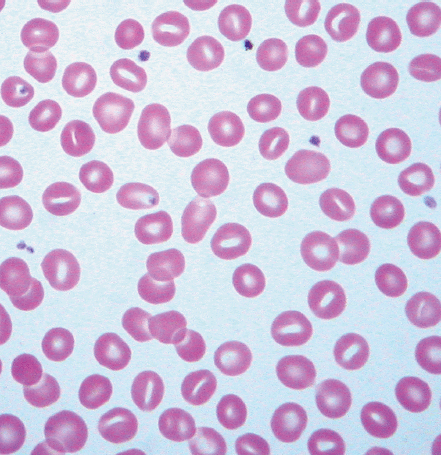
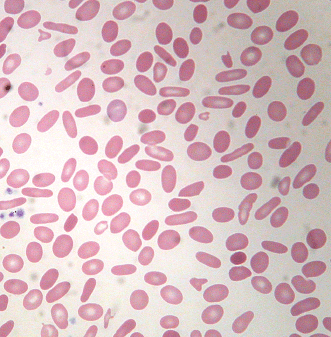
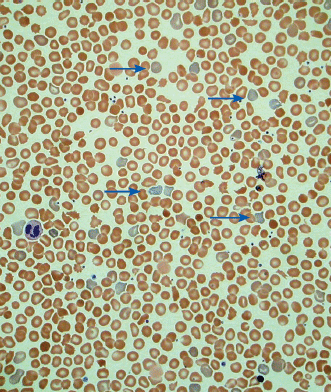
Identifying the cause of anemia is usually fairly straightforward. There are several strategies for reaching the diagnosis (Tables 10–2 and 10–3), 1 of which is illustrated in the algorithms (Figures 10–1 to 10–4). Examination of the peripheral smear is especially important, since numerous clues can be found there (Table 10–4). Figures 10–5 to 10–24 show many of the abnormal morphologies and intracellular inclusions of RBCs.
| Production Defect | Survival Defect |
|---|---|
|
|
|
|
| Normal RDW | High RDW | |
|---|---|---|
| Low MCV |
|
|
| Normal MCV |
|
|
| High MCV |
|
|
Figure 10–4
Diagnostic algorithm for suspected hemolytic anemia. DIC, disseminated intravascular coagulation; TTP, thrombotic thrombocytopenic purpura; HUS, hemolytic uremic syndrome; HELLP, hemolysis, elevated liver function tests, and low platelets; HS, hereditary spherocytosis; HE, hereditary elliptocytosis; G6PD, glucose-6-phosphate dehydrogenase.
| Finding | Definition | Associated Conditions |
|---|---|---|
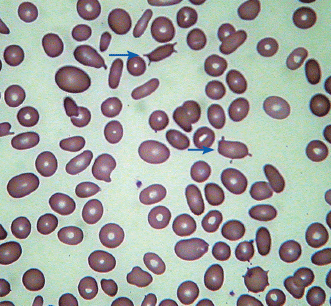
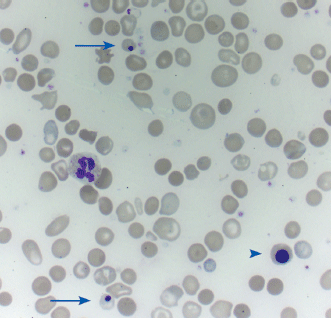
| Basophilic stippling | Small blue dots in red cells, due to clusters of ribosomes |
|
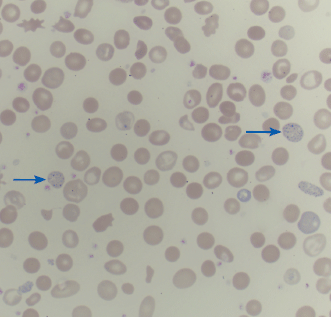
| Pappenheimer bodies | Larger, more irregular, and grayer than basophilic stippling, due to iron-containing mitochondria |
|
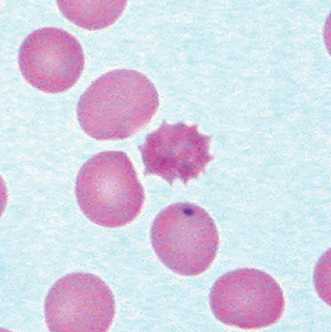
| Heinz bodies Bite cells | Heinz bodies: gray-black round inclusions, seen only with supravital stains (crystal violet). Bite cells: sharp bite-like defects in red cells where a Heinz body has been removed in the spleen. Both are due to denatured hemoglobin |
|
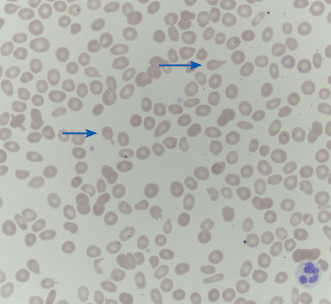
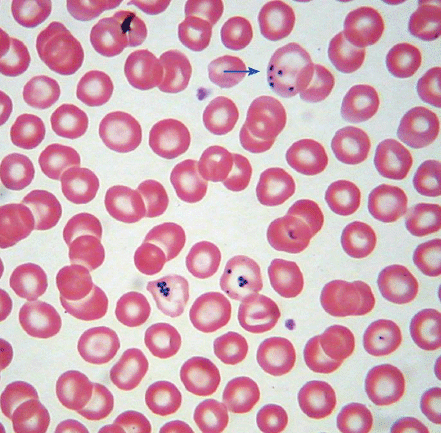
| Howell–Jolly bodies Cabot rings | Howell–Jolly body: dot-like, dark purple inclusion. Cabot ring: ring-shaped dark purple inclusion. Both represent a residual nuclear fragment |
|
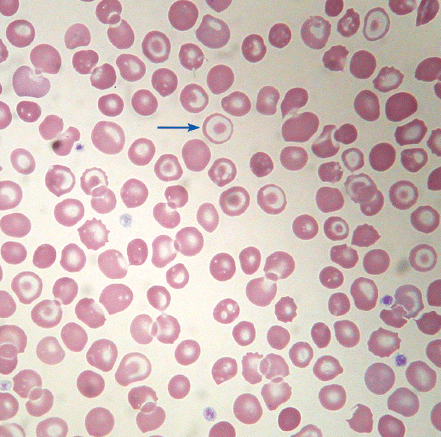
| Target cells | Red cells with a dark circle within the central area of pallor, reflecting redundant membrane |
|
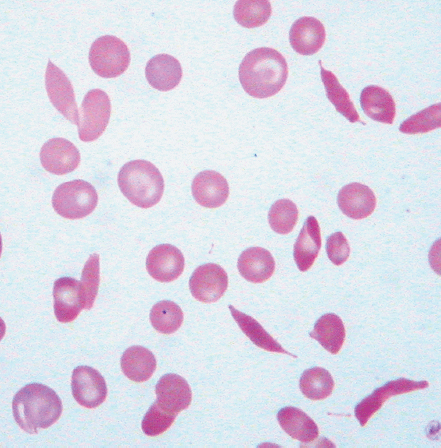
| Schistocytes | Fragmented red blood cells, with forms such as helmet-shaped cells, due to mechanical red cell fragmentation |
|
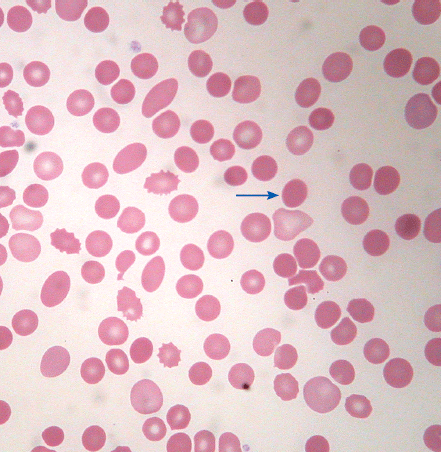
| Dacrocytes (teardrop cells) | Teardrop or pear-shaped erythrocytes |
|
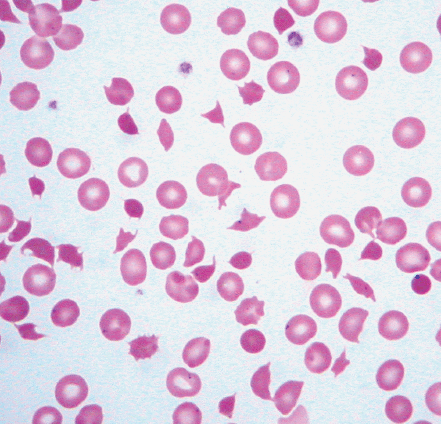
| Echinocytes (burr cells) | Red blood cells that have circumferential undulations or spiny projections with pointed tips |
|
| Acanthocytes (spur cells) | Red blood cells that have circumferential blunt and spiny projections with bulbous tips |
|
| Spherocytes | Red cells without central pallor due to decreased red cell membrane |
|
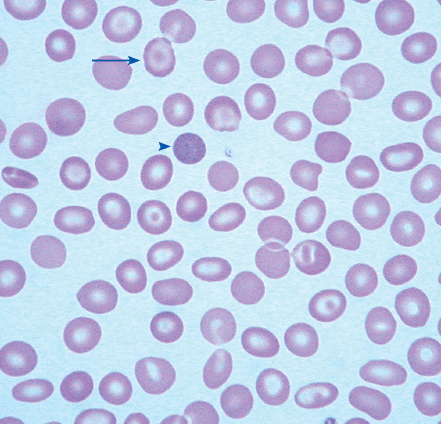
| Elliptocytes | Red cells twice as long as they are wide |
|
| Stomatocytes | Red cells whose area of central pallor is elongated in a mouth-like shape |
|
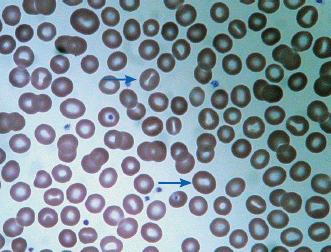
The reticulocyte count is an important piece of information. When markedly elevated, this is usually noticeable in a Wright-stained peripheral blood smear. Reticulocytes appear as large, polychromatophilic red cells, and when they are numerous, the smear is often described as having polychromasia. Anemia due to a production defect is associated with a normal to low reticulocyte count. Such hyporegenerative anemias include iron deficiency anemia, anemia of chronic disease (ACD), lead poisoning, folate deficiency, B12 deficiency, myelodys-plastic syndrome, aplastic anemia, and pure red cell aplasia.
Regardless of the morphology or red cell size, anemia that is accompanied by reticulocytosis suggests either hemolysis or hemorrhage. Some exceptions should be noted. One is a partially treated production defect, such as in the early treatment of iron, folate, or B12 deficiency, in which one may find persistent anemia with reticulocytosis. Second, both hemolytic and blood-loss anemia may eventually lead to depletion of iron, folate, or B12, and they can present as a production defect. Lastly, paroxysmal nocturnal hemoglobinuria (PNH) is a hemolytic anemia that may transform to aplastic anemia.
Hemolytic anemias are those in which red cell survival, normally 120 days, is shortened. The premature destruction of erythrocytes may occur within the bloodstream (intravascular hemolysis) or within the reticuloendothelial system (eg, extravascular hemolysis). Intravascular hemolysis is caused by mechanical red cell trauma (microangiopathic hemolytic anemia [MHA] from mechanical heart valve), complement fixation on the red cell surface (eg, ABO incompatibility), paroxysmal nocturnal hemoglobinuria (PNH), paroxysmal cold hemoglobinuria (PCH), snake envenomation, and infectious agents (eg, malaria, babesiosis, Clostridium). Extravascular hemolysis is much more common and is typical for all remaining causes of hemolysis. The causes of hemolysis may be inherited or acquired. Inherited forms of hemolytic anemia usually, but not always, present in early childhood (Table 10–5).
| Intravascular Hemolysis | Extravascular Hemolysis |
|---|---|
| Schistocytes | Microspherocytes |
| ↑ Lactate dehydrogenase (LD) | ↑ LD |
| ↓ Haptoglobin | Normal to ↓ haptoglobin |
| ↑ Free hemoglobin, ↑ urine hemoglobin | ↑ Indirect bilirubin |
| Hemosiderinuria | ↑ Urine and fecal urobilinogen |
Hemolytic anemia presents with jaundice, fatigue, tachycardia, and pallor. Enhanced excretion of Hb breakdown products often leads to the development of pigmented gallstones. Intravascular hemolysis may present with dark urine and back pain. Leg ulcers are common in sickle cell disease and hereditary spherocytosis (HS). Splenomegaly is a common finding in extravascular hemolysis. Laboratory findings in support of hemolysis include increased unconjugated bilirubin, increased lactate dehydrogenase (LD), and decreased haptoglobin. Reticulocytes, which are larger than mature red cells, are responsible for an unpredictability of the mean corpuscular volume (MCV). The blood smear may display helpful morphologic findings. Intravascular hemolysis is associated with hemoglobinuria and hemosiderinuria.
Acute blood loss (hemorrhage) is seen most often as a result of surgery, trauma, or gastrointestinal pathology. Most often, hemorrhage is quite obviously present, but occasionally it is occult and internal (large retroperitoneal or pelvic hemorrhages). It can occur in the prehospital setting, and in that case its volume cannot be estimated.
Acute blood loss (hemorrhage) is seen most often as a result of surgery, trauma, or gastrointestinal pathology.
The cardinal manifestations of acute blood loss—tachycardia, tachypnea, and hypotension—reflect not so much a decreased oxygen-carrying capacity as a decreased intravascular volume. A shift of water from the interstitial fluid compartment into the plasma leads to hemodilution and a lowered hematocrit (hct). It is for this reason that the initial treatment is intravenous fluid resuscitation with normal saline; only if this is unsuccessful is blood transfusion considered.
Chronic slow blood loss is generally well tolerated and usually presents late in the disease process as iron deficiency anemia. Acute blood loss is not the only form of anemia that can present abruptly. Causes other than hemorrhage that may present as rapid-onset severe anemia include intravascular hemolysis and acute exacerbations of a chronic compensated hemolytic anemia, such as in sickle cell disease (Table 10–6).
| Acute Intravascular Hemolysis | Acute Exacerbation of Chronic Hemolysis |
|---|---|
| Microangiopathic hemolytic anemia | Parvovirus B19 bone marrow infection (aplastic crisis) |
| Mechanical hemolysis (eg, heart valve) | Splenic sequestration crisis |
| Toxins (eg, venoms) | Hyperhemolytic crisis |
| Infections (eg, malaria, Clostridium) | |
| Oxidant stress (especially in glucose-6-phosphate dehydrogenase deficiency) | |
| Hemolytic transfusion reaction (ABO incompatibility) | |
| Paroxysmal nocturnal hemoglobinuria | |
| Paroxysmal cold hemoglobinuria |
The history and physical examination are the keys to arriving at the correct diagnosis. In perplexing situations, it may be necessary to exclude hemolysis. The main laboratory findings are a normocytic anemia with a marked reticulocytosis. The peripheral smear may be notable only for neutrophilia, a result of mobilization of granulocytes from marginal pools (demargination), which is a physiologic stress response. Somewhat later, there may be reactive thrombocytosis.
Within the cytoplasm of the marrow erythroblast, the predominant activity is the production of Hb molecules into which iron must be incorporated. Iron from the diet is absorbed principally in the duodenum. It is carried by transferrin to the marrow where it is internalized into erythroblasts and incorporated into protoporphyrin to yield heme. Iron not utilized in this way is stored bound to ferritin. When there is inadequate iron intake or excessive iron loss (Table 10–7), the ferritin–iron stores of the reticuloendothelial system become progressively depleted. Red cells are produced that contain an inadequate concentration of Hb, giving rise to the appearance of small, hypochromic red cells that are poorly equipped for the carriage of oxygen. Fewer mature red cells are subsequently produced, lowering the Hct (Table 10–8). The clinical manifestations include those directly attributable to anemia (fatigue, pallor), in addition to pica (a desire to ingest solids such as rock, dirt, or ice), atrophic glossitis, koilonychias, and esophageal webs. The coexistence of esophageal webs and iron deficiency has been called Plummer–Vinson syndrome. These latter manifestations are not commonly seen and follow prolonged, untreated iron deficiency.
| Stage | Laboratory Findings | Clinical Findings |
|---|---|---|
| Iron store depletion | ↓ Serum ferritin, ↓ stainable marrow iron | None |
| Impaired erythropoiesis | All of the above plus ↑ TIBC, ↓ serum iron, and ↑ RDW | None |
| Iron deficiency anemia | All of the above plus microcytic, hypochromic anemia | Fatigue, pallor |
Iron deficiency is the most common cause of anemia. Worldwide, the most common cause of iron deficiency is a dietary lack of iron. In the United States, iron intake is not usually problematic, although supply can lag demand in some populations, such as toddlers and pregnant women. The finding of iron deficiency produces an obligation to identify and treat the underlying cause. In American adults, this underlying cause is usually found within the gastrointestinal tract. Iron deficiency often is the first sign of an occult gastrointestinal malignancy.