Diffuse Large Cell Lymphoma of the Mediastinum
Key Facts
Clinical Issues
Predominantly affects women in 3rd decade of life
Presents with rapidly growing, large (> 10 cm in diameter), infiltrative anterior mediastinal mass
Rarely involves peripheral lymph nodes, bone marrow, or other lymphoid organs
Commonly presents with superior vena cava syndrome, pleural effusion, and airway obstruction
Relapses involve a variety of extrathoracic sites, including kidneys, adrenals, liver, and central nervous system
Microscopic Pathology
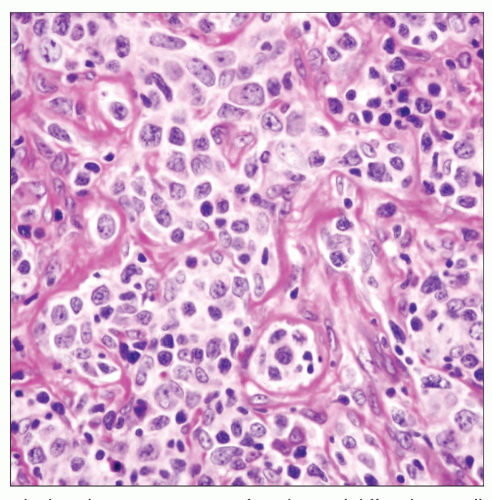
Nuclei may show great variability, from centroblastic to immunoblastic morphology
Nuclear pleomorphism with bizarre forms can often be found
Crushing artifact is common finding in small endoscopic biopsies or core needle biopsies
Stromal sclerosis may be fine and delicate or coarse, separating tumor cells into small nests
Consistent B-cell phenotype: CD19(+), CD20(+), CD22(+), CD79-α(+)
Other markers: May show CD30(+) when using heat-induced epitope retrieval techniques; generally Bcl-6(+), variably Bcl-2(+)
Negative markers: Ig, CD5, CD15, CD21, CD30 (in multinucleated tumor cells), EBV
Other lymphoid markers: CD45(+), HLA-DR(+), IgG or IgA(+), may show κ or λ light chain restriction
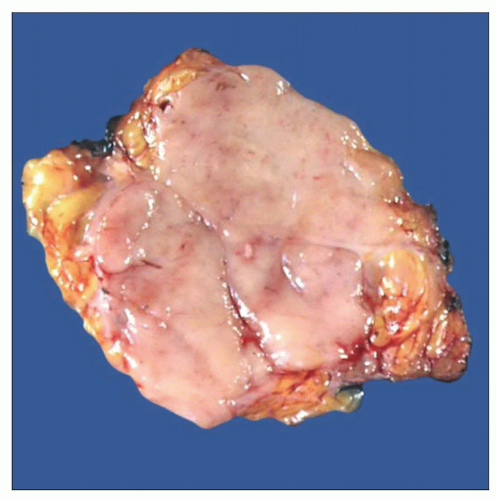 Gross photograph of mediastinal diffuse large cell lymphoma shows an ill-defined mass with tan-white, lobulated, and homogeneous cut surface. |
TERMINOLOGY
Abbreviations
Diffuse large cell lymphoma of the mediastinum (DLCLM)
Synonyms
Mediastinal large B-cell lymphoma (MLBCL); mediastinal diffuse large cell lymphoma (MDLCL); diffuse large cell lymphoma with sclerosis
Definitions
Primary large cell lymphoma arising from native thymic lymphoid B-cell population
ETIOLOGY/PATHOGENESIS
Pathogenesis
Derived from extranodal, mucosa-associated, native lymphoid cell population originating in medullary compartment of thymus
In some cases, may represent progression from extranodal marginal zone B-cell lymphoma (MALToma) of thymus
Gene expression profiling has demonstrated 30% similarity of gene expression with classical Hodgkin lymphoma
CLINICAL ISSUES
Epidemiology
Incidence
Represents about 6% of all large cell lymphomas in adults
Represents about 25% of all childhood non-Hodgkin lymphomas
Age
Most commonly occurs in young adults (range: 15-73; median: 32)
Can also occur in children and adolescents
Gender
Predilection for women
Presentation
Distinctive clinicopathologic syndrome
Predominantly affects women in 3rd decade of life
Presents with rapidly growing, large (> 10 cm in diameter), infiltrative anterior mediastinal mass
Rarely involves peripheral lymph nodes, bone marrow, or other lymphoid organs
Commonly presents with superior vena cava syndrome, pleural effusion, and airway obstruction
Relapses involve variety of extrathoracic sites, including kidneys, adrenals, liver, and central nervous system
Treatment
Combination chemotherapy ± radiation therapy
Prognosis
Despite high-grade histology and aggressive presentation, DLCLMs respond well to combination chemotherapy
Recent studies have shown remission rates of nearly 80% and disease-free survival of 60% at 3 years
IMAGE FINDINGS
Radiographic Findings
Mediastinal widening with pleural effusion
CT Findings
Large mediastinal mass obscuring borders of heart and great vessels
Infiltration of lung or pericardium
Pleural effusion
MACROSCOPIC FEATURES
General Features
Bulky mediastinal disease with infiltration of lung, pleura, pericardium, and chest wall
Homogeneous rubbery cut surface; may show areas of hemorrhage and necrosis
MICROSCOPIC PATHOLOGY
Histologic Features
Most cases are characterized by sheets of large lymphoid cells with prominent nucleoli and pale cytoplasm
May show unusual histologic features, including following subtypes
Clear cell mediastinal lymphoma
Sheets of large CD20(+) lymphoid cells with large nuclei and prominent nucleoli surrounded by ample rim of water-clear cytoplasm
Clear cell lymphoma may resemble metastases of renal cell carcinoma and other clear cell malignant neoplasms
Spindle cell mediastinal lymphoma
Sheets or fascicles of CD20(+) spindle cells with elongated, hyperchromatic nuclei
May show a prominent “storiform” pattern resembling malignant fibrohistiocytic neoplasms
Spindle cell lymphoma may be confused for a variety of spindle cell sarcomas
Pleomorphic mediastinal large cell lymphoma
Sheets of bizarre CD20(+) pleomorphic tumor cells with atypical multinucleated and multilobated cells
Pleomorphic large cell lymphoma may resemble variety of high-grade pleomorphic sarcomas
Large cell mediastinal lymphoma with marked tropism for germinal centers (“germinotropic lymphoma”)
Clusters of large, CD20(+) atypical lymphoid cells encroaching on germinal centers and replacing follicular center cells
Irregular replacement of follicles by large, atypical lymphoid cells in fashion reminiscent of progressive transformation of germinal centers
Atypical lymphoid cells are found within lymphoid follicles without involvement of interfollicular areas
Germinotropic lymphoma may be confused for mediastinal seminoma, metastatic carcinoma, or melanoma
Signet ring cell mediastinal lymphoma
Sheets or singly scattered CD20(+) lymphoid cells displaying prominent signet-ring cell features are seen admixed with lymphoid cells
Signet-ring cell lymphoma may be confused for liposarcoma or metastases of signet-ring cell carcinoma
Mediastinal large B-cell lymphoma with Hodgkin-like or anaplastic large cell lymphoma-like features
Sheets of large anaplastic CD20(+) lymphoid cells with indented nuclei and prominent eosinophilic nucleoli
Sheets of Reed-Sternberg-like multinucleated or multilobated B-lymphoid cells with prominent eosinophilic nucleoli
Tumor cells may be positive for CD30 but are also positive for CD20 and are negative for ALK1 and T-cell markers
Diffuse large B-cell lymphoma with sclerosis
Nests of large, atypical CD20(+) lymphocytes surrounded by variable degrees of stromal collagenization, creating a “compartmentalized” growth pattern
Stromal sclerosis may be fine and delicate or coarse, separating tumor cells into small nests
Advanced stages of collagenization may show extensive paucicellular stromal sclerosis with few residual atrophic tumor cells
Nests of tumor cells separated by fibrous septa may be confused for carcinoma or melanoma
Other unusual histologic features
Residual thymic epithelial islands may show hyperplastic features and undergo cystic changes
Sheets of atypical B lymphocytes may show degenerative changes, resulting in “pseudoalveolar” growth pattern
Tumor cells may show angiocentric distribution around vessel walls with plugging of vessel wall lumen
Some cases show overlapping morphologic and genetic features with Hodgkin lymphoma (so-called gray-zone lymphoma)
Cytologic Features
Nuclei may show great variability, from centroblastic to immunoblastic morphology
Nuclear pleomorphism with bizarre forms can often be found
Reed-Sternberg-like cells and small multinucleated or multilobated cells can also be seen
Cells can be spindled, signet-ring, clear, or pleomorphic
Crushing artifact is common finding in small endoscopic biopsies or core needle biopsies
Tumors involving thymus may contain entrapped benign thymic epithelial elements
Tumor cell necrosis is frequent finding in large tumors
Tumor cells often show extensive infiltration of adjacent structures, with sparing and preservation of mediastinal fat
ANCILLARY TESTS
Immunohistochemistry
Consistent B-cell phenotype: CD19(+), CD20(+), CD22(+), CD79-α(+)
Other lymphoid markers: CD45(+), HLA-DR(+), IgG, or IgA(+)
Other markers: May show weak CD30 positivity when using heat-induced epitope retrieval techniques; generally Bcl-6(+), variably Bcl-2(+)
Negative markers: Ig, EBV, CD5, CD15, CD21, CD30 (in multinucleated tumor cells)
Immunoexpression of MAL is seen in 50% of cases
May show κ or λ light chain restriction
Molecular Genetics
Frequent chromosomal gains: 2p, 6p, 7q, 9p, 12, and X
Most important cytogenetic abnormality is gain of chromosome arm 9p observed in up to 75% of cases, leading to overexpression of c-REL
Aberrations of chromosome X are seen in up to 87% of cases
Bcl-2 rearrangements observed in up to 30% of cases
Overexpression of MAL gene is believed to be specific for MDLCL although it is shared with classic Hodgkin lymphoma
Immunoglobulin gene rearrangements are present
Absence of Epstein-Barr virus (EBV) genome
Gene expression profiling shows close similarities with classical Hodgkin lymphoma
DIFFERENTIAL DIAGNOSIS
Thymic Carcinoma and Metastatic Carcinoma
Tumor cells are cohesive and positive for epithelial markers (cytokeratins, etc.) in thymic carcinoma
Metastatic renal cell carcinoma is positive for cytokeratins, EMA, CD10, and vimentin
Thymic carcinoma and metastatic carcinoma tend to occur at older age
Anaplastic Large Cell Lymphoma (ALCL)
Sclerosis and compartmentalization are not features of ALCL
Immunophenotype of ALCL is CD30(+), ALK1(+)
Staining for CD30 in neoplastic cells is stronger and more diffuse in ALCL than in MDLCL
ALCL is characterized by t(2;5) translocation
Hodgkin Lymphoma
Mixed cell infiltrate with eosinophils, plasma cells, and small lymphocytes
Reed-Sternberg cells are CD15/CD30(+) and negative for CD19, CD20, and CD22
Metastatic Amelanotic Malignant Melanoma
Melanoma cells are positive for S100 protein and other melanocytic markers (HMB-45, Melan-A, tyrosinase, etc.)
Melanoma cells are negative for CD20 and CD45
Mediastinal Seminoma
Tumor cells have large vesicular nuclei with prominent irregular nucleoli due to presence of nucleolonemata
Tumor cells are positive for PLAP, OCT4, and D2-40, and negative for CD20 and CD45
Sarcoma
Usually occurs in older age group than MDLCL
Pleomorphic sarcomas are negative for lymphoid markers (CD20, CD45)
DIAGNOSTIC CHECKLIST
Clinically Relevant Pathologic Features
Age distribution
Young adults (3rd-4th decades)
Gross appearance
Large bulky masses with extensive infiltration of local structures (lung, pleura, pericardium, diaphragm, etc.)
Metastatic distribution
Unusual distribution of extrathoracic metastases: Kidneys, adrenals, liver, pancreas, ovaries, CNS
Pathologic Interpretation Pearls
Core biopsies are often difficult to diagnose due to distortion by sclerosis or necrosis
Care must be taken not to misinterpret keratin-positive, entrapped, residual thymic epithelium for evidence of carcinoma on small biopsies
Nested, compartmentalized appearance should not be mistaken for other types of tumors
High level of suspicion should exist in proper clinicopathologic context
Young female
Rapidly growing and extensively invasive anterior mediastinal mass
No peripheral lymphadenopathy
Immunohistochemical stains are critical for diagnosis
CD20/CD45(+)
May be weakly and focally positive for CD30 using heat-induced epitope retrieval
Negative for CD5, CD10, CD15, CD21
Negative for other markers of differentiation (keratins, CEA, EMA, S100 protein, HMB-45, PLAP, etc.)
Overexpression of MAL gene is seen in 50% of cases and is considered specific for MDLCL, but it can also be observed in 30% of nodular sclerosing Hodgkin lymphoma
Molecular studies may be of limited value
No rearrangements of T-cell receptor β chain gene constant region
No distinctive cytogenetic chromosomal translocations
May show Ig heavy or light chain gene rearrangements
Absence of t(2;5) may help to distinguish MDLCL from ALCL in equivocal cases
SELECTED REFERENCES
1. Faris JE et al: Primary mediastinal large B-cell lymphoma. Clin Adv Hematol Oncol. 7(2):125-33, 2009
2. Fietz T et al: Treatment of primary mediastinal large B cell lymphoma with an alternating chemotherapy regimen based on high-dose methotrexate. Ann Hematol. 88(5):433-9, 2009
3. Dunphy CH et al: Primary mediastinal B-cell lymphoma: detection of BCL2 gene rearrangements by PCR analysis and FISH. J Hematop. 1(2):77-84, 2008
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree