Cytology
The utility of cytology is almost totally dependent upon the cytologist’s interest integrating the clinical and histological information. Without this interest, cytology usually adds very little information or may even be misleading because of false-positive diagnoses or the frequent diagnosis of “suspicious for malignancy.” Cytology has gained increasing importance with the advent of EUS and passage of needles via EUS into mass lesions and lymph nodes. In fact, in many EUS centers, a designated cytology assistant is present in the endoscopy suite to perform the smears and confirm that analyzable cells are present. By far its most frequent use is in lesions of the pancreatico-biliary tree.
Direct-vision brush cytology. Direct-vision brush cytology can enhance diagnostic accuracy in malignancy when it is used as an adjunct to biopsy.
9 Sometimes brush cytology is the only technique available to establish a diagnosis of malignancy, especially when there are very tight strictures of the esophagus, stomach, or colon.
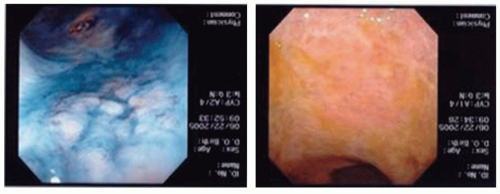
Balloon mesh cytology. Balloon mesh cytology (
Fig. 1-7) is used to screen for squamous carcinoma in high-risk groups.
10
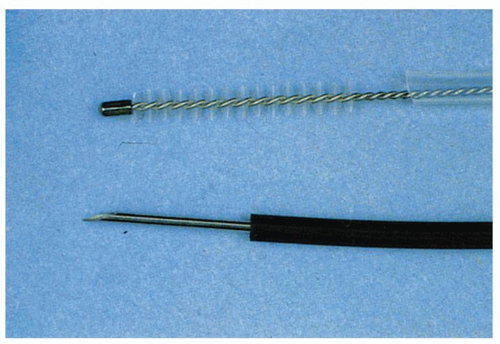
Fine-needle aspirates. Fine-needle aspirates can be obtained from thickened folds or submucosal lesions. This is done by passing a needle attached to hollow plastic tubing through the biopsy channel of the endoscope (
Fig. 1-5). The needle is pushed into the wall, suction is applied with a syringe attached to the proximal end of the plastic sheath, and the needle is jiggled back and forth two or three times. The needle is then retracted into the sheath and removed from the endoscope, and the contents are blown onto a slide. A second slide is apposed, and a smear is prepared just as for a blood smear. It is useful to repeat this procedure one or two more times so that four to six slides are available for cytology review. Experience with this technique has grown with the advent of EUS for the evaluation of submucosal lesions, for infiltrative disorders (e.g., lymphoma) associated with a normal overlying mucosa, and for assessing nodes adjacent to tissues accessible at EUS (i.e., mediastinum, paragastric, paraduodenal, celiac axis, paracolic, and pararectal).
Chromoendoscopy
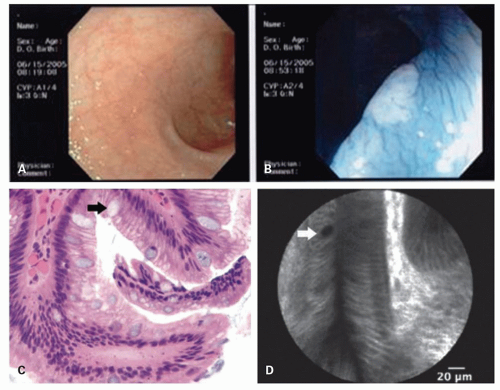
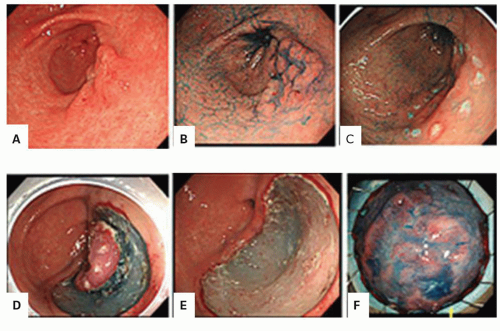
Chromoendoscopy (the use of dye stains or an image system that changes the color visualized at endoscopy to enhance visualization of potential neoplastic lesions) either with a magnifying endoscope or without has been increasingly explored as an approach in both Barrett’s esophagus and inflammatory bowel disease (IBD) colitis dysplasia screening (
Fig. 1-8). Methylene blue as chromogen is taken up by actively absorbing intestinal-type epithelial cells and dysplastic cells but not by squamous or gastric mucosa. A lighter intensity of staining would highlight an area of dysplasia. So while methylene blue was introduced as an agent to distinguish inflammation from normal colonic mucosa in the mid-1980s,
11,
12 it has been studied as an adjunctive technique for identifying neoplastic from nonneoplastic mucosa in the esophagus
and the colon. Missing some foci of inflammation in an otherwise obviously inflamed organ has much less clinical implication than missing neoplastic foci in either an inflamed or a noninflamed organ. Indigo carmine as chromogen enhances the mucosal surface by pooling in the grooves between the mucosal villi enabling the visualization of the pattern formed by the mucosal folds and pits. Acetic acid achieves the same goal by means of reversible desaturation of superficial mucosal proteins. The bottom line is that these dyes or acetic acid can accentuate mucosal pit patterns so that neoplastic ones become more evident.
Because the spraying of solutions can be time consuming and messy, the advent of narrow band imaging (NBI) had the potential to be a major advance. NBI involves light of a short wavelength (blue light in the visible spectrum) penetrating superficially into the mucosa allowing for improved surface detail. As blue light is highly absorbed by hemoglobin, the vascular pattern is especially accentuated. The major advantages of NBI are that it involves merely the switch of a button on the head of the endoscope and hence requires little time and also lacks mess. Further it is uniformly applied, whereas dye spraying can be nonuniform.
Autofluorescence imaging (AFI) uses blue light for excitation of endogenous tissue fluorophores which emit fluorescent green light of longer wavelength. It can also highlight neoplastic tissue without the need for exogenous fluorophores. These latter two modalities have the potential advantage of not just identifying neoplasia when present that might be missed by white light endoscopy, but also by highlighting the superficial pit patterns of the lesion in question. This could help the endoscopist identify if a lesion is in fact neoplastic or is merely hyperplastic.
Endoscopes are currently available that are tri-modal. These scopes have the usual high-definition white light endoscopy with buttons that allow conversion to either NBI or AFI.
13 These imaging systems are already widely available, whereas more sophisticated modalities such as confocal microscopy remain mostly available in research centers.
Confocal endomicroscopy allows visualization of individual cells and their nuclei, so that the enlarged stratified nuclei of dysplasia may be directly identified. While chromoendoscopy with dye spraying or other novel imaging systems exist, do they actually improve the detection of neoplasia in any of Barrett’s esophagus, chronic colitis, or simply for routine screening or surveillance colonoscopy in the search for adenomas or dysplasia? This is discussed subsequently.
Regardless of how they are identified, whenever a neoplastic or presumed neoplastic lesion is removed and when there is a question of having to return sooner rather than later for repeat endoscopic assessment, it is prudent for the endoscopist to inject India ink (Spot) at the site of lesion removal. This helps for subsequent targeted biopsies either during surveillance or to provide a map for the surgeons who will resect that area. Endoscopists must notify pathologists that biopsies were performed from previously tattooed areas.
Barrett’s esophagus. Methylene blue staining was reported to be highly accurate in identifying dysplasia in Barrett’s esophagus.
14 In one report, methylene blue enhanced the detection of the extent of the Barrett’s but not necessarily of finding dysplastic lesions, most of which were visible endoscopically.
15,
27 In another Barrett’s esophagus study, methylene blue staining was only 37% sensitive in picking up dysplastic lesions compared with routine histological assessment of four-quadrant biopsies. Although the specificity was good in this latter study (97%), to obviate the need for multiple biopsies, sensitivity would have to be excellent.
16 Therefore high rates of predictive value of methylene blue for identifying dysplasia are either operator dependent or may require specialized magnifying endoscopes. In one randomized crossover study of methylene blue versus random four-quadrant biopsy in patients with dysplasia in Barrett’s found methylene blue directed biopsies to be significantly less sensitive in detecting dysplasia than routine biopsies in Barrett’s esophagus.
17 Methylene blue identified dysplasia in 9 of 18 subjects while random biopsy with white light endoscopy found dysplasia in 17 of 18 leading these authors to suggest that methylene
blue dye spray with targeted biopsies was inferior to random nontargeted biopsies. Further, a higher percentage of biopsies in the random biopsy group were dysplastic (36%) compared to that of the methylene blue targeted group (26%,
p = 0.05).
17 A meta-analysis of nine studies comparing methylene blue chromoendoscopy with routine white light endoscopy plus biopsy revealed no incremental benefit of methylene blue chromoendoscopy over white light endoscopy.
18
A series of studies have explored the role of NBI for detecting dysplasia in Barrett’s esophagus. One of the first observational studies showed a benefit of using NBI in defining nondysplastic and high-grade dysplastic Barrett’s epithelium.
19 In a prospective tandem study of 65 patients with Barrett’s esophagus, NBI identified more subjects with higher grades of dysplasia than white light endoscopy (18% vs. 0), while standard endoscopy was associated with more biopsies (8.5 vs. 4.7,
p < 0.001).
20 While NBI is easy to use, a study of 8 endoscopists scoring 1,600 NBI images of Barrett’s esophagus found moderate interobserver agreement at best, including for high high-grade dysplasia. This suggests that NBI could not replace histological evaluation for neoplasia in Barrett’s.
21 Perhaps the most sobering of studies for enhanced imaging in Barrett’s esophagus was a study comparing white light endoscopy with the enhancement of any of indigo carmine chromoendoscopy, acetic acid chromoendoscopy, and NBI. Twelve endoscopists examined 22 areas, all assessed with the four techniques. The chromoendoscopy techniques added nothing to the interobserver agreement achieved on white light endoscopy. So while endoscopists appreciated the enhancement techniques as revealing more appealing images, the images did not enhance clinical outcomes over and above white light endoscopy.
22Inflammatory bowel disease. The first randomized controlled trial of dysplasia surveillance in IBD was performed in Germany, where approximately half the subjects underwent routine dysplasia surveillance and half underwent dysplasia surveillance with chromoendoscopy.
23 Chromoendoscopy was performed by spraying methylene blue 0.1% on the colonic mucosa in 30-cm segments and observing the mucosa with a special magnifying endoscope. Biopsies were directed to the paler, less blue, or white areas since neoplastic epithelium is less likely to take up the dye. During the course of the chromoendoscopy, the mucosa was scored for inflammation. The investigators found that chromoendoscopy better delineated the extent of inflammation by an average of 14 cm. More importantly, chromoendoscopy identified significantly more neoplastic lesions (35 in 13 cases, of which 32 were dysplastic and 3 were cancers, vs. routine endoscopy plus biopsy where neoplasia was detected 11 times in 6 cases, of which 10 were dysplastic and 1 was cancer). However, chromoendoscopy did not identify significantly more
patients with dysplasia than routine endoscopy. Chromoendoscopy did lead to some false positives and even a few false negatives.
Overall the Kiesslich study has been considered a success of chromoendoscopy in ulcerative colitis (UC) which may relate simply to the use of methylene blue, which could be widely adopted by endoscopists with little extra cost. However, based on the experience with Barrett’s esophagus, it may have reflected the use of the specialized magnifying endoscopes in combination with the dye. The availability of this technique may be dependent on the availability of these specialized endoscopes, and their widespread purchase will likely depend on further validation of this method. Elsewhere, the application of indigo carmine dye immediately after a standard surveillance endoscopy in 100 patients enhanced the yield of finding dysplasia in 2 patients to 7 in UC.
24The dye spraying technique may help target biopsies, so considerably fewer biopsies will be necessary. This technique may also help solve the problem of identifying at endoscopy those lumps that are neoplastic and not simply inflammatory; this would be a major advance. However, in colons widely studded with inflammatory (pseudo)polyps, the effects of the dye may be obscured.
23 False positives are also evident with chromoendoscopy as both highly inflamed areas and neoplastic areas do not take up the methylene blue dye.
23 Thus, chromoendoscopy will likely not aid in the dilemma of distinguishing dysplasia histologically in the setting of severe inflammation.
Some studies evaluating dye spraying did not employ a magnifying endoscope or mucolytics. The potential of chromoendoscopy without magnification endoscopy was evaluated in 102 chronic colitis patients undergoing surveillance. Each patient underwent two passes of the colonoscope; the first pass involved random biopsies with targeted biopsies of raised lesions. The second pass was after spraying of methylene blue dye and targeted biopsies of only raised or suspicious lesions. The dye spray method yielded 17 patients with dysplasia versus 9 with the targeted nondye technique and only 3 with the random biopsy technique. This group advocated consideration of abandoning random biopsies in favor of targeted biopsying only as directed by dye spraying.
25 In practice, chromoendoscopy, as described without a magnifying endoscope, can identify raised lesions that may only be inflammatory and not neoplastic (
Fig. 1-8) but may also accentuate raised lesions that are neoplastic facilitating more focused biopsies (
Fig. 1-9). Chromoendoscopy is typically not helpful with widespread inflammatory (pseudo) polyps (
Fig. 1-10).
NBI identifies vascular changes and is very good at identifying the extent of inflammation (areas that
are endoscopically normal may actually have subtle vascular changes and NBI may identify this). It may not be as effective at identifying mass lesions as dye-based chromoendoscopy, although this requires further study. In one study, 50 patients with UC were endoscopically inspected with a scope that had the capacity for usual white light endoscopy, NBI, as well as autofluorescence—AFI (trimodal endoscopy).
26 NBI can help determine the extent of inflammation and hence target where increased biopsy sampling should be undertaken. It is not clear that NBI is as good as dye spraying for colitis-associated neoplasia.