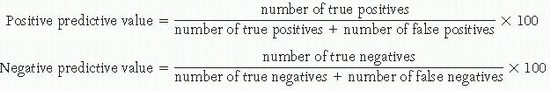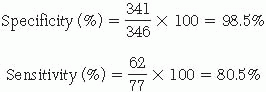OVERVIEW OF THE CLINICIAN’S ROLE: RESPONSIBILITIES, STANDARDS, AND REQUISITE KNOWLEDGE
In this era of high technology, health care delivery involves many different disciplines and specialties. Consequently, clinicians must have an understanding and working knowledge of modalities other than their own area of expertise. This includes diagnostic evaluation and diagnostic services. Laboratory and diagnostic tests are tools to gain additional information about the patient. By themselves, these tests are not therapeutic; however, when used in conjunction with a thorough history and physical examination, these tests may confirm a diagnosis or provide valuable information about a patient’s status and response to therapy that may not be apparent from the history and physical examination alone. Generally, an evidenced-based tiered approach to selecting tests is used:
Basic screening (frequently used with wellness groups and case finding)
Establishing (initial) diagnoses
Differential diagnosis
Evaluating current medical case management and outcomes
Evaluating disease severity
Monitoring course of illness and response to treatment
Group and panel testing
Regularly scheduled screening tests as part of ongoing care
Testing related to specific events, certain signs and symptoms, or other exceptional situations (e.g., infection and inflammation [bladder infection or cellulitis], sexual assault, drug screening, pheochromocytoma, postmortem tests, to name a few) (
Table 1.1)
Test selections are based on subjective clinical judgment, national recommendations, and evidence-based health care. Often, diagnostic tests or procedures are used as predictors of surgical risk or morbidity and mortality rates because, in some cases, the risk may outweigh the benefit.
Recent national surveys have revealed that most individuals are not knowledgeable about recommendations for primary screening tests used to detect various types of cancers. Many times, patients pressure their physicians to order various test procedures as well as treatment. Patients sometimes feel that more is better in terms of laboratory tests. The potential for the clinician to order unwarranted tests, or “blanket testing,” has resulted in significant cost to the health care system. Multiplex testing—conducting multiple tests on a single sample—however, can be beneficial under the right circumstances, such as with autoimmune disorders or genetically inherited diseases, and in the long run can be cost saving. Use of evidence-based guidelines for scheduling, selecting, retaining, or eliminating certain diagnostic tests may help achieve more effective case management and cost containment. These guidelines use a system that grades the quality of scientific evidence based on published reports of clinical trials, expert consensus, or clinical expertise. Levels of evidence are A to C and E, with A being the best evidence and E referring to expert opinion or consensus (
Chart 1.1).
As an integral part of their practice, clinicians have long supported patients and their significant others in meeting the demands and challenges incumbent in the simplest to the most complex diagnostic testing. This testing begins before birth and frequently continues after death. Prenatal testing may include ultrasound, genetic testing, and amniocentesis. Postmortem testing can be done for evidentiary or forensic purposes, organ transplantation, or death reporting (autopsy). Clinical responsibilities and interventions cover all three phases of the testing process: the pretest, intratest, and posttest periods. The clinician who provides diagnostic services must have basic requisite knowledge to plan patient
care and an understanding of psychoneuroimmunology (effects of stress on health status), make careful judgments, and gather vital information about the patient and the testing process to diagnose appropriately within the parameters of the clinician’s professional standards (
Table 1.2;
Chart 1.2).
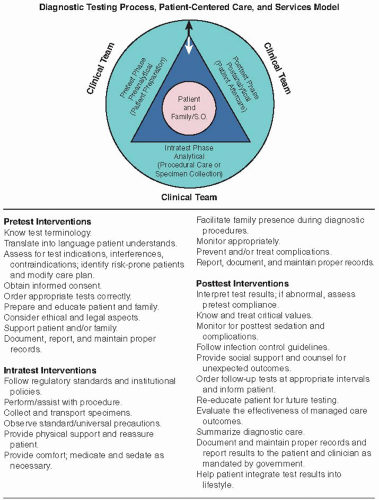
The diagnostic testing model incorporates three phases: pretest, intratest, and posttest (
Fig. 1.1).
The clinical team actively interacts with the patient and his or her significant others throughout each phase. The following components are included with each laboratory test or diagnostic procedure in this text:
Pretest Interventions:
Test background information
Reference (normal) values
Explanation of test
Indications for testing
Signs, symptoms, and history of disease
Intratest Interventions:
Actual description of procedures
Proper specimen collection and transport
Gathering and using right equipment
Clinical implications of abnormal results
Interfering factors that clinician or patient can or cannot control (e.g., age, gender) and avoid (e.g., medications)
Posttest Interventions:
Patient monitoring
Clinical and procedure alerts
Special cautions
Interpretation of test results
Treatment, medical, surgery, follow-up
Each phase of testing requires that a specific set of guidelines and standards be followed for accurate, optimal test results. Patient care standards and standards of professional practice are key points in developing a collaborative approach to patient care during diagnostic evaluation. Standards of care provide clinical guidelines and set minimum requirements for professional practice and patient care. They protect the public against less-than-quality care (
Table 1.3).
If test results are inconclusive or negative and no definitive medical diagnosis can be established, other tests and procedures may be ordered. Thus, testing can become an involved and lengthy process. In the future, digital pathology and computerized algorithms may be helpful to the clinician when it comes to diagnosis and directed treatment.
Understanding the basics of safe, effective, and informed care is important. These basics include assessing risk factors and modifying care accordingly, using a collaborative approach, following proper guidelines for procedures and specimen collection, and delivering appropriate care throughout the process. Providing reassurance and support to the patient and his or her significant others, intervening appropriately, and clearly documenting patient teaching, observations, and outcomes during the entire process are important (see
Fig. 1.1).
A risk assessment before testing identifies risk-prone patients and helps to prevent complications. The following factors increase a patient’s risk for complications and may affect test outcomes:
Age ≥65 years: decrease/increase for expected test outcomes
History of falls
History of serious chronic illnesses
History of allergies (e.g., latex, contrast iodine, radiopharmaceuticals, and other medications)
Infection or increased risk for infection (e.g., HIV, organ transplantation, chemotherapy, radiation therapy)
Aggressive or antisocial behavior
Seizure disorders
Uncontrolled pain
Gastric motility dysfunction
Use of assistive devices for activities of daily living (ADLs)
Unsteady gait, balance problems
Neuromuscular conditions
Weakness, fatigability
Paresthesias
Impaired judgment or illogical thinking
Severe visual problems
Hearing impairment
Use of diuretics, sedatives, analgesics, or other prescription or over-the-counter (OTC) drugs
Alcohol or illegal drug use or addiction
The environments in which diagnostic services are provided, the degree of cultural diversity present in the community, and the physical, emotional, social, and spiritual state of the patient all influence the patient’s response to the procedure. Including the patient’s significant others is a vital component of the entire process and must not be taken lightly or casually dismissed.
New and emerging changes are occurring in the health care field, such as telemedicine, personalized medicine (using one’s own genetic makeup to manage their disease), and nanomedicine (treating disease at the molecular level). In the future, online resources (e.g., eMedicine) may be used by the physician where traditional clinical resources may be limited. New guidelines focus on evidence-based practice—for example, using pH test papers to confirm correct nasogastric tube placement (pH <4.0) rather than having to rely on an x-ray procedure. When a patient is admitted to the emergency department with chest pain, a whole blood specimen for cardiac troponin is collected on arrival and 6 hours later, thereby replacing CK-MB measurement. Recent Laboratory Medicine Practice Guidelines (LMPGs) have addressed diabetes, liver tumor markers, pharmacogenetics, and newborn screening (National Academy of Clinical Biochemistry, American Association for Clinical Chemistry). The emerging field of metabolomics (analysis of metabolites) has been increasingly used to identify biomarkers.
Although patient populations and testing environments vary, the potential contagions are universal and of concern for both the patient and the health care worker.
Standard and universal precautions must be used in all testing environments to ensure safety for patients and clinicians. Certain tests
(e.g., cholesterol screening, blood glucose, electrocardiogram [ECG], lipid profiles, tuberculosis [TB] skin tests) can be done in the field, meaning that the service is brought to the patient’s environment. Other tests (e.g., x-rays using contrast media and those that require special patient preparation, invasive procedures, nuclear medicine procedures, hormone levels, and 24-hour urine testing panels) must be done in a physician’s office, clinic, or hospital setting. Magnetic resonance imaging (MRI) and ultrasound procedures (e.g., echocardiograms) are commonly performed in freestanding or specialty diagnostic centers. Complex tests such as endoscopic retrograde cholangiopancreatography (ERCP), cardiac catheterization, and bronchoscopy may require hospital admission or at least outpatient status. As testing equipment becomes more technologically sophisticated and risks associated with testing are reduced, the environment in which diagnostic procedures take place will also shift. Insurance reimbursement for testing also influences trends. Managed care and case management, together with collaboration among the diverse health care disciplines and the patient, are key factors in determining how and to what degree optimal diagnostic services are used. Clear, timely, accurate communication among all patients and professionals is key to minimizing problems and frustrations.
As societies become more culturally blended, the need to appreciate and work within the realm of cultural diversity becomes imperative. Interacting with patients and directing them through diagnostic testing can present certain challenges if one is not familiar with and sensitive to the health care belief system of the patient and his or her significant others. Something as basic as attempting to communicate in the face of language differences may necessitate arrangements for a relative or translator to be present during all phases of the process. Special attention and communication skills are necessary for these situations as well as when caring for children and for comatose, confused, or frail patients. Consideration of these issues will significantly influence compliance, outcomes, and positive responses to the procedure. To be most effective, professional care providers must be open to a holistic perspective and attitude that affects their caregiving, communication, and patient-empowering behaviors. Clinicians who understand the patient’s basic needs and expectations and strive to accommodate those as much as possible are truly acting as patient advocates.
Preparing patients for diagnostic or therapeutic procedures, collecting specimens, carrying out and assisting with procedures, and providing follow-up care have long been requisite activities of professional practice. This care may continue even after the patient’s death. Diagnostic postmortem services include death reporting, possible postmortem investigations, and sensitive communication with grieving families and significant others regarding autopsies, unexplained death, other postmortem testing, and organ donation (see
Chapter 16).
Professionals need to work as a team to meet diverse patient needs, to facilitate certain decisions, to develop comprehensive plans of care, and to help patients modify their daily activities to meet test requirements in all three phases. It is given that institutional protocols are followed.
PRETEST PHASE: ELEMENTS OF SAFE, EFFECTIVE, INFORMED CARE
The emphasis of pretest care is on appropriate test selection, obtaining proper consent, proper patient preparation, individualized patient education, emotional support, and effective communication. These interventions are essential to achieving the desired outcomes and preventing misunderstandings and errors.
Basic Knowledge and Necessary Skills
Know the test terminology, purpose, process, procedure, and normal test reference values or results. The names of diseases are a convenient way of briefly stating the end point of a diagnostic process that begins with assessment of symptoms and signs and ends with knowledge of causation and detection of underlying disorders of structure and function.
The clinical value of a test is related to its sensitivity, its specificity, and the incidence of the disease in the population tested. Sensitivity and specificity do not change with different populations of ill and healthy patients.
True positive: positive test result in a person with the disease True negative: negative test result in a person without the disease False positive: positive test result in a person without the disease False negative: negative test result in a person with the disease
Specificity refers to the ability of a test to identify correctly those individuals who do not have the disease. The formula for specificity is as follows:
Sensitivity refers to the ability of a test to correctly identify those individuals who truly have the disease. The formula for sensitivity is as follows:
Incidence refers to the number of new cases of a disease, during a specified period of time, in a specified population or community.
Prevalence refers to the number of existing cases of a disease, at a specific period of time, in a given population.
Predictive values refer to the ability of a screening test result to correctly identify the disease state. The predictive value of the same test can be very different when applied to people of differing ages, gender, geographic locations, and cultures. True-positive results correctly identify individuals who actually have the disease, and true-negative results correctly identify individuals who do not actually have the disease. Positive predictive value equals the percentage of positive tests with true-positive results (i.e., the individual does have the disease). Negative predictive value refers to the percentage of negative tests with true-negative results (i.e., the individual does not have the disease).
See
Table 1.4 for an example that demonstrates the specificity, sensitivity, and predictive values for a new screening test to identify the cystic fibrosis gene.
Thus, this new screening test will give a false-negative result about 20% of the time (e.g., the person does have the cystic fibrosis gene but his or her test results are negative).
Thus, there is about an 8% chance that the person will test positive for the cystic fibrosis gene but does not have it.
Thus, there is about a 5% chance that the person will test negative for the cystic fibrosis gene but actually does have it.
Look at both current and previous test results and review the most recent laboratory data first, then work sequentially backward to evaluate trends or changes from previous data. The patient’s plan of care may need to be modified because of test results and changes in medical management.
Testing Environments
Diagnostic testing occurs in many different environments. Many test sites have shifted into community settings and away from hospitals and clinics.
Point-of-care testing (POCT) refers to medical testing done in close proximity to the site of patient care (e.g., in the primary care setting or acute care settings [emergency room, critical care units, ambulances]), thus moving toward decentralized testing. Recently, manufacturers of POCT devices have made inroads into physician offices, off-site clinics, and pharmacies. POCT is convenient for the patient, produces rapid reporting of test results, and allows for more timely assessment, management, and treatment.
Testing in the home care environment requires skill in procedures such as drawing blood samples, collecting specimens from retention catheters, proper specimen labeling, documentation, specimen handling, and specimen transporting. Moreover, teaching the patient and his or her significant others how to collect specimens is an important part of the process.
In occupational environments, testing may be done to reduce or prevent known workplace hazards (e.g., exposure to lead) and to monitor identified health problems. This can include pre-employment baseline screening, periodic monitoring of exposure to potentially hazardous workplace substances, and drug screening. Skill in drawing blood samples, performing breathing tests, monitoring chain of custody (see
Chapter 3), and obtaining properly signed and witnessed consent forms for drug, genetic, and HIV testing is required.
More pretest, posttest, and follow-up testing occurs in nursing homes and long-term care facilities because patients are frequently taken or transferred to hospitals for more complex procedures (e.g., computed tomography [CT] scans, endoscopies), whereas this is not the case with routine testing. Increasing numbers of full code (i.e., resuscitation) orders lead to greater numbers and varieties of tests. Additionally, confused, combative, or uncooperative behaviors are seen more frequently in these settings. An attitude adopted by nursing home patients of not wanting to be bothered or engaging in outright refusal to undergo prescribed tests can make testing difficult. Consequently, understanding
patient behaviors and using appropriate communication strategies and interventions for this population are necessary skills for practicing in this arena.
For those who practice in the realm of public health, diagnostic test responsibilities focus on wellness screenings, preventive services, disease control, counseling, and treatment of individuals with problems. Case finding frequently occurs at health fairs, outreach centers, homeless shelters, neighborhood nurse offices, mobile health vans, and church settings. Responsibilities vary according to setting and may include providing test information, procuring specimens, and recommending referrals to appropriate caregivers. These responsibilities may even extend to transporting and preparing specimens for analysis or actually performing specimen analysis (e.g., stool tests for occult blood, TB skin testing, and procuring blood or saliva samples for HIV/AIDS testing).
History and Assessment
Obtain a relevant, current health history; perform a physical assessment if indicated. Identify conditions that could influence the actual testing process or test outcomes (e.g., pregnancy, diabetes, cultural diversity, language barrier, physical impairment, altered mental state).
Perform a risk assessment for potential injury, adverse event, or noncompliance.
Identify contraindications to testing such as allergies (e.g., iodine, latex, medications, contrast media). Records of previous diagnostic procedures may provide clues.
Assess for coping styles and knowledge or teaching needs.
Assess fears and phobias (e.g., claustrophobia, panic attack, fear of needles and blood). Ascertain what strategies the patient uses to deal with these reactions and try to accommodate these.
Observe standard/universal precautions with every patient (see
Appendix A). A patient may choose not to disclose drug or alcohol use or HIV and hepatitis risks.
Document relevant data. Address patient concerns and questions. This information adds to the database for collaborative problem-solving activities among the medical, laboratory-diagnostic, and nursing disciplines.
Reimbursement for Diagnostic Services
Differences in both diagnostic care services and reimbursement may vary between private and government insurance. Nonetheless, quality of care should not be compromised in favor of cost reduction. Advocate for patients regarding insurance coverage for diagnostic services. Inform the patient and his or her family or significant others that it may be necessary to check with their insurance company before laboratory and diagnostic testing to make certain that costs are covered.
Many insurance companies employ case managers as gatekeepers for monitoring costs, diagnostic tests ordered, and other care. As a result, the insurance company or third-party payer may reimburse only for certain tests or procedures or may not cover tests considered by them to be preventive care. So that reimbursement completely covers diagnostic services provided, be sure to include proper documentation (e.g., date of laboratory service and specimen collected) and proper current procedural terminology (CPT) codes.
Chart 1.3 lists laboratory tests that are covered by most insurance carriers, both private and government.
Methodology of Testing
Follow testing procedures accurately. Verify orders and document them with complete, accurate, and legible information. Document all drugs the patient is taking because these may influence test outcomes (see
Appendix E).
Ensure that specimens are correctly obtained, preserved, handled, labeled, and delivered to the appropriate department. For example, it is not generally acceptable to draw blood samples when an intravenous line is infusing proximal to the intended puncture site.
Observe precautions for patients in isolation. Use standard/universal precautions or other transmission-based precautions as dictated by infection control policies of the institution.
Health care personnel should protect themselves, as appropriate, through the use of personal protective equipment (PPE). Standards have been developed and implemented in an effort to prevent the transmission of blood-borne pathogens from patients to health care workers. Training and education can only be effective when health care professionals are continually diligent about safety.
As much as possible, coordinate patient activities with testing schedules to avoid conflicts with meal times and administration of medications, treatments, or other diagnostic tests and travel time.
Maintain NPO (i.e., Latin: non per os, nothing by mouth) status when necessary.
Administer the proper medications in a timely manner. Schedule tests requiring contrast substances in the proper sequence so as not to invalidate succeeding tests.
Interfering Factors
Minimize test outcome deviations by following proper test protocols. Make certain the patient and his or her significant others know what is expected of them. Written instructions are very helpful.
Reasons for deviations may include the following:
Incorrect specimen collection, handling, storage, or labeling
Wrong preservative or lack of preservative
Delayed specimen delivery
Incorrect or incomplete patient preparation
Hemolyzed blood samples
Incomplete sample collection, especially of timed samples
Old or deteriorating specimens
Patient factors that can alter test results may include the following:
Incorrect pretest diet
Current drug therapy
Type of illness
Dehydration
Position or activity at time of specimen collection
Postprandial status (i.e., time patient last ate)
Time of day
Pregnancy
Level of patient knowledge and understanding of testing process
Stress
Nonadherence or noncompliance with instructions and pretest preparation
Undisclosed drug or alcohol use
Age and gender
Avoiding Errors
To avoid costly mistakes, know what equipment and supplies are needed and how the test is performed. Communication errors account for more incorrect results than do technical errors. Properly identify and label every specimen as soon as it is obtained. With the use of bar code technology, transcription error rates have substantially decreased and data entry is much quicker and more accurate. Determine the type of sample needed and the collection method to be used. Additionally, the following should also be considered:
Is the test invasive or noninvasive?
Are contrast media injected or swallowed?
Is there a need to fast?
Are fluids restricted or forced?
Are medications administered or withheld?
What is the approximate length of the procedure?
Are consent forms properly completed?
Is a local anesthetic, conscious sedation, oxygen, analgesia, or anesthesia required?
Report test results as soon as possible. Critical values must be reported immediately (STAT, Latin: statim) to the appropriate health care provider (e.g., physician, nurse, or physician assistant [PA]), depending on institutional policy.
Instruct patients and their significant others regarding their responsibilities. Accurately outline the steps of the testing process and any restrictions that may apply. Conscientious, clear, timely communication among health care departments can reduce errors and inconvenience to both staff and patients.
Proper Preparation
Prepare the patient correctly. This preparation begins at the time of scheduling and extends to the testing facility.
Provide information about the testing site and give directions for locating the facility; allow time to enter the facility and find the specific testing laboratory. If a copy of the written test order was given to the patient to bring to the laboratory, interpret the test order.
Plan to be at the department 15 minutes before testing if the test is scheduled for a specific time. Upon arrival, the patient needs to be properly identified by at least two identifiers (e.g., asking the patient to spell their last name and providing their date of birth). Once proper identification has been confirmed, the patient should review any preprinted labels that may be used to label lab specimens and/or wristband if appropriate. Review all pretest instructions and be certain they are explained clearly (e.g., explain to the patient what fasting actually means if the patient is given fasting directions for a test).
Be cognizant of special needs of the patient with physical limitations or disabilities, ostomies, or diabetes; children; elderly patients; and culturally diverse patients.
Give simple, accurate, precise instructions (scripted) according to the patient’s level of understanding. For example, the patient needs to know when and what to eat and drink or how long to fast.
Encourage dialogue about fears and apprehensions. “Walking” a patient through the procedure using imagery and relaxation techniques may help the patient to cope with anxieties. Never underestimate the value of a caring presence.
Assess for the patient’s ability to read and understand instructions. Poor eyesight or hearing difficulties may impair understanding and compliance. Speak slowly and clearly. Do not bombard the patient with information. Instruct the patient to use assistive devices such as eyeglasses and hearing aids if necessary. Clear, written instructions can reinforce verbal instructions and should be used whenever possible. In some cases, a translator, sign language interpreter, or legal representative may be necessary.
Assess for language and cultural barriers. Patients behave according to personal values, perceptions, beliefs, traditions, and cultural and ethnic influences. Take these into consideration and value the patient’s uniqueness to the highest degree possible.
Document accurately in all testing phases.
Patient Education
Educate the patient and family regarding the testing process and what will be expected of them. Record the date, time, type of teaching, information given, and person to whom the information was given.
Giving sensory and objective information that relates to what the patient will likely physically feel and the equipment that will be used is important so that patients can envision a realistic representation of what will occur. Avoid technical and medical jargon and adapt information to the patient’s level of understanding. Slang terms may be necessary to get a point across.
Encourage questions and verbalization of feelings, fears, and concerns. Do not dismiss, minimize, or invalidate the patient’s anxiety through trivial remarks such as “Don’t worry.” Develop listening skills and be aware of nonverbal signals (i.e., body language) because these frequently provide a more accurate picture of what the patient really feels than what he or she says. Above all, do not be judgmental.
Emphasize that there is usually a waiting period (i.e., turn-around time) before test results are relayed back to the clinicians and nursing unit. The patient may have to wait several days for results. Offer listening, presence, and support during this time of great concern and anxiety.
Record test result information. Include the patient’s response. Just because something is taught does not necessarily mean that it is learned or accepted. The possibility that a diagnosis will require a patient to make significant lifestyle changes (e.g., diabetes) requires intense support, understanding, education, and motivation. Document specific names of audiovisual and reading materials to be used for audit, reimbursement, and accreditation purposes.
Testing Protocols
Develop consistent protocols for teaching and testing that encompass comprehensive pretest, intratest, and posttest care modalities.
Prepare patients for those aspects of the procedure experienced by most patients. Clinicians can collaborate to collect data and to develop a list of common patient experiences, responses, and reactions.
Patient Independence
Allow the patient to maintain as much control as possible during the diagnostic phases to reduce stress and anxiety. Include the patient and his or her significant others in decision making. Because of factors such as anxiety, language barriers, and physical or emotional impairments, the patient may not fully understand and assimilate instructions and explanations. To validate the patient’s understanding of what is presented, ask the patient to repeat instructions given to evaluate assimilation and understanding of presented information.
Include and reinforce information about the diagnostic plan, the procedure, time frames, and the patient’s role in the testing process.
Test Results
It is important to understand normal or reference values/intervals and ranges.
Normal ranges can vary to some degree from laboratory to laboratory. Frequently, this is because of the particular type of equipment used. Theoretically, normal can refer to the ideal health state, to average reference values or intervals, or to types of statistical distribution. Normal values are those that fall within two standard deviations (i.e., random variation) of the mean value for the normal population. The reference interval typically represents the upper and lower limits wherein 95% of healthy people would fall. Although establishing normal or reference values is complex, it is much more so in the pediatric population. There are many challenges related to sex- and age-specific reference intervals in the pediatric population.
The reported reference range for a test can vary according to the laboratory used, the method employed, the population tested, and methods of specimen collection and preservation.
Most normal blood test values are determined by measuring fasting specimens.
Specific factors can influence test results. For example, patient posture is important when plasma volume is measured because this value is 12% to 15% greater in a person who has been supine for several hours. Changing from a supine to a standing position can alter values as follows: increased hemoglobin (Hb), red blood cell (RBC) count, hematocrit (Hct), calcium (Ca), potassium (K), phosphorus (P), aspartate aminotransferase (AST), phosphatases, total protein, albumin, cholesterol, and triglycerides. Going from an upright to a supine position results in increased hematocrit, calcium, total protein, and cholesterol. A tourniquet applied for more than 1 minute produces laboratory value increases in protein (5%), iron (6.7%), AST (9.3%), and cholesterol (5%) and decreases in K+ (6%) and creatinine (2% to 3%).
Laboratories must specify their own normal ranges. Many factors affect laboratory test values and influence ranges. Thus, values may be normal under one set of prevailing conditions but may exhibit different limits in other circumstances. Age, gender, race, environment, posture, diurnal and other cyclic variations, foods, beverages, fasting or postprandial state, drugs, and exercise can affect derived values. Interpretation of laboratory results must always be in the context of the patient’s state of being. Circumstances such as hydration, nutrition, fasting state, mental status, or compliance with test protocols are only a few of the situations that can influence test outcomes.
Laboratory Reports
Scientific publications and many professional organizations are changing clinical laboratory data values from conventional units to Système International (SI) units. Currently, many data are reported in both ways.
The SI system uses seven dimensionally independent units of measurement to provide logical and consistent measurements. For example, SI concentrations are written as amount per volume (moles or millimoles per liter) rather than as mass per volume (grams, milligrams, or milliequivalents per deciliter, 100 milliliters, or liter). Numerical values may differ between systems or may be the same. For example, chloride is the same in both systems: 95 to 105 mEq/L (conventional) and 95 to 105 mmol/L (SI).
Converting to Système International Units
Clinical laboratory data may be reported in conventional units, SI units, or both. Examples of conversion of data from the two systems are included in
Table 1.5. To convert SI units to conventional U.S. units,
divide by the factor; to convert conventional US units to SI units,
multiply by the factor.
Example:
To convert a digoxin (drug management) level of 0.6 nmol/L (SI units), divide by the factor 1.281 to obtain conventional units of 0.5 ng/dL.
To convert a Ca2+ (electrolyte) value of 8.6 mg/dL (conventional units), multiply by the factor 0.2495 to obtain the SI units of 2.15 mmol/L.
Margins of Error
Recognize margins of error. For example, if a patient has a battery of chemistry tests, the possibility exists that some tests will be abnormal owing purely to chance. This occurs because a significant margin of error arises from the arbitrary setting of limits. Moreover, if a laboratory test is considered normal up to the 95th percentile, then 5 times out of 100, the test will show an abnormality even though a patient is not ill. A second test performed on the same sample will probably yield the following: 0.95 × 0.95, or 90.25%. This means that 9.75 times out of 100, a test will show an abnormality even though the person has no underlying health disorder. Each successive testing will produce a higher percentage of abnormal results. If the patient has a group of tests performed on one blood sample, the possibility that some of the tests will read abnormal due purely to chance is not uncommon.
Ethics and the Law
Consider legal and ethical implications. These include the patient’s right to information, correct diagnosis and prognosis, properly signed and witnessed consent forms, and explanations and instructions regarding chain-of-custody requirements and risks as well as benefits of tests.
Chain of custody is a legal term descriptive of a procedure to ensure specimen integrity from collection, to transport, to receipt, to analysis and specimen storage. A special form is used to provide a written record. The right to informed consent before certain tests and procedures pertains to patient autonomy, the ethical right of self-determination, the legal right to be free of procedures to which one does not consent, and the right to determine what will be done to one’s own person. Risks, benefits, and alternatives are explained and written consent obtained well in advance of the procedure.
The patient must demonstrate appropriate cognitive and reasoning faculties to sign a legally valid consent. Conversely, a patient may not legally give consent while under the immediate influence of sedation, anesthetic agents, or certain classes of analgesics and tranquilizers. If the patient cannot validly and legally sign a consent form, an appropriately qualified individual may give consent for the patient.
Guidelines and wishes set forth in advance directives or living will-type documents must be honored, especially in life-threatening situations. Such directives may prevent more sophisticated invasive procedures from being performed. Some states have legislated that patients can procure do-not-resuscitate (DNR) orders and medical DNR bracelets that indicate their wishes. A copy of a patient’s advance directives in the health care record can be very helpful in unpredictable situations.
A collaborative team approach is essential for responsible, lawful, and ethical patient-focused care. The clinician who orders the test has a responsibility to inform the patient about risks and test results and to discuss alternatives for follow-up care. Other caregivers can provide additional information and clarification and can support the patient and family in achieving the best possible outcomes. The duty to maintain confidentiality, to provide freedom of choice, and to report infectious diseases may result in ethical dilemmas. For example, a dilemma to be faced in diagnostic testing surrounds HIV screening: Should it be risk based or routine?
When faced with ethical dilemmas, choosing the correct course of action is a challenge. The responsibility to provide safe, effective care may conflict with the ethics of privacy, confidentiality, quality of life, and patient safety. The Health Insurance Portability and Accountability Act (HIPAA) of 1996 has set forth regulations regarding patient confidentiality. Protected health information (PHI) is addressed under HIPAA regulations and includes any information—whether oral, written, electronic, or recorded in any manner—that is created or received by a health care worker; relates to the patient’s past, present, or future health care management; identifies the patient (e.g., Social Security number, medical record number); or can be used to identify a patient. HIPAA also provides rights to patients (e.g., the right to request, amend, correct, or limit their PHI).