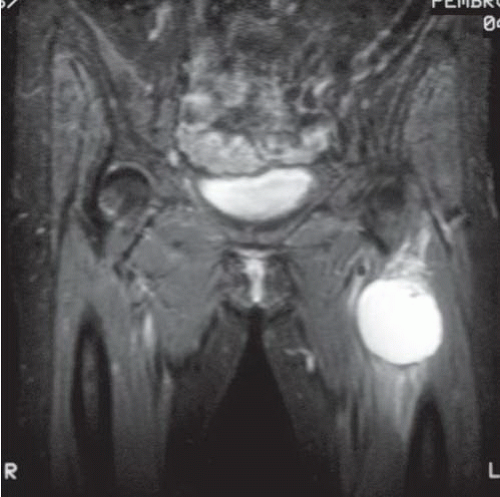
typically appears first. In most reported cases, the fibrous dysplasia involves more than one bone (polyostotic) and, in some instances, can be associated with Albright syndrome (polyostotic fibrous dysplasia, café au lait spots on the skin, and endocrine abnormalities). The intramuscular myxomas typically arise in the same general vicinity as the affected bone(s). Intramuscular myxoma is a benign tumor that rarely recurs following local excision.
TABLE 21.1 The Big Three: Myxoid Liposarcoma, Intramuscular Myxoma, and Myxofibrosarcoma/Myxoid Malignant Fibrous Histiocytoma | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 21.2 Differential Diagnosis of Deep Myxoid Lesions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
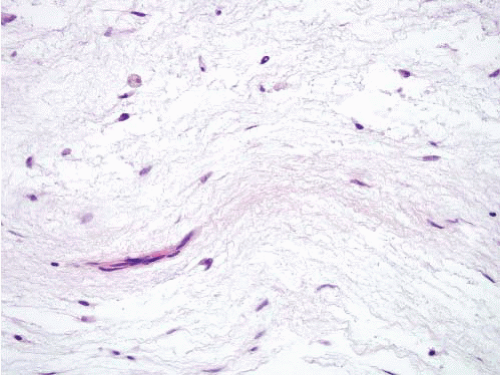
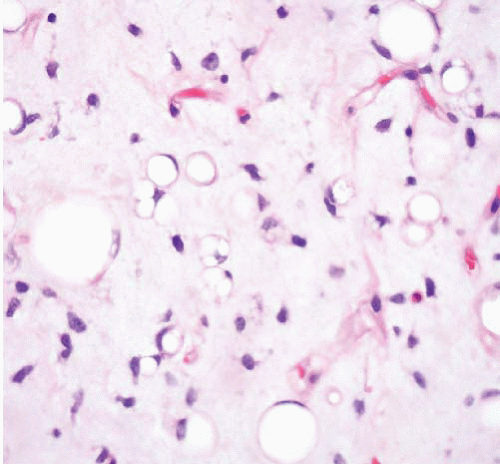
process. At the periphery of the lesion, skeletal muscle fibers adjacent to the tumor are atrophic and separated by edema fluid or infiltrating tumor. Fat cells are commonly interspersed in the skeletal muscle.
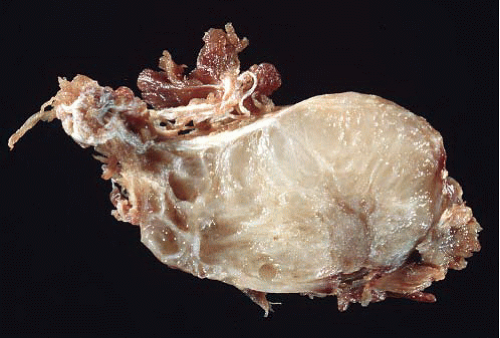 FIGURE 21.2 Gross image from an intramuscular myxoma. The tumor is overall wellcircumscribed with a glairy cut surface. |
cutaneous and cardiac myxomas in patients with Carney complex (spotty skin pigmentation, cardiac and other myxomas, and different types of endocrine tumors), whose lesions have PRKAR1A alterations.7
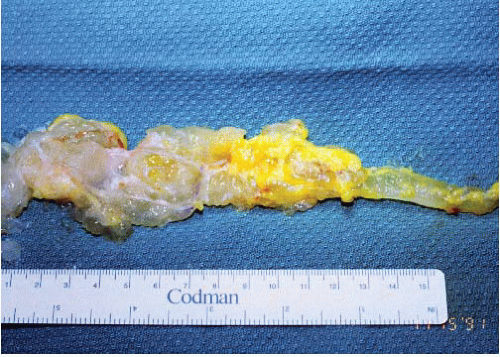 FIGURE 21.4 Juxta-articular myxoma. This lesion arose in the wrist of a young woman— an unusual site (most occur near large joints such as the knee joint). |
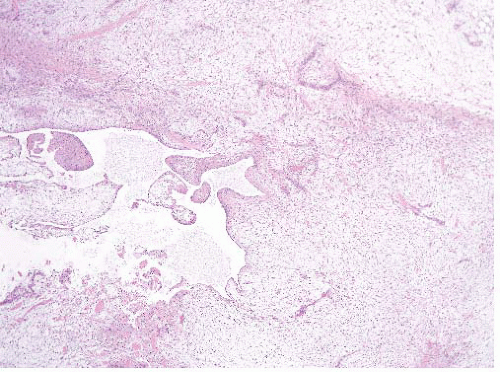 FIGURE 21.5 Juxta-articular myxoma. These tumors often have cystic spaces. Note that this lesion is far more cellular than the intramuscular myxoma seen in Figure 21.3. |
ground substance. The majority of the tumor cells have scant cytoplasm and uniform dark-staining nuclei. Nucleoli or mitotic figures are typically not prominent. Since myxoid liposarcoma is a translocation-associated sarcoma, it tends to have uniform cells with essentially no nuclear pleomorphism and no abnormal mitoses.18 This feature makes it easy to distinguish from myxofibrosarcoma/myxoid MFH, which has pleomorphic cells (and is also usually more superficial; see Table 21.2). On needle biopsies, the classic features can become obscured either by crush artifact or sampling error and well-formed lipoblasts are not apparent (e-Figs. 21.10 to 21.13). In these cases, attention to the vascularity and uniform cytologic features can be helpful. At times, smears stained with Romanowsky stains can disclose lipoblasts that are otherwise inapparent (e-Fig. 21.14).
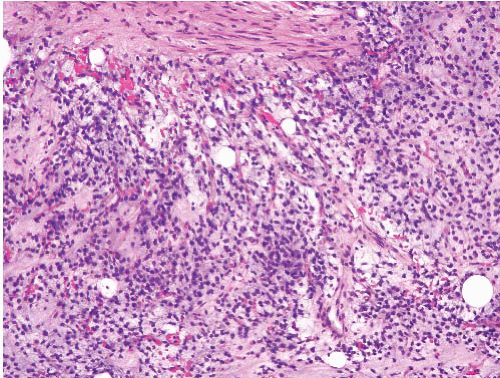 FIGURE 21.6 Myxoid liposarcoma. This myxoid liposarcoma features uniform round nuclei, a rich capillary network, and scattered lipoblasts. |