Abbreviations and Acronyms
| Ab | Antibody |
| Abn | Abnormal |
| AFB | Acid-fast bacillus |
| Ag | Antigen |
| AIDS | Acquired immunodeficiency syndrome |
| ALT | Alanine aminotransferase |
| ANA | Antinuclear antibody |
| AST | Aspartate aminotransferase |
| CBC | Complete blood cell count |
| CF | Complement fixation |
| CHF | Congestive heart failure |
| CIE | Counterimmunoelectrophoresis |
| CK | Creatine kinase |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CXR | Chest x-ray |
| CYP | Cytochrome P450 |
| Diff | Differential cell count |
| EDTA | Ethylenediaminetetraacetic acid (edetate) |
| ELISA | Enzyme-linked immunosorbent assay |
| GI | Gastrointestinal |
| GNR | Gram-negative rod |
| GNCB | Gram-negative coccobacillus |
| GPC | Gram-positive coccus |
| GVCB | Gram-variable coccobacillus |
| HLA | Human leukocyte antigen |
| Ig | Immunoglobulin |
| IM | Intramuscular(ly) |
| INR | International Normalized Ratio |
| IV | Intravenous(ly) |
| Min | Minute |
| MN | Mononuclear cell |
| MRI | Magnetic resonance imaging |
| N | Normal |
| Neg | Negative |
| NPO | Nothing by mouth (nil per os) |
| PCR | Polymerase chain reaction |
| PMN | Polymorphonuclear neutrophil (leukocyte) |
| PO | Orally (per os) |
| Pos | Positive |
| PTH | Parathyroid hormone |
| RBC | Red blood cell |
| RPR | Rapid plasma reagin (syphilis test) |
| SIADH | Syndrome of inappropriate antidiuretic hormone (secretion) |
| SLE | Systemic lupus erythematosus |
| T3 | Triiodothyronine |
| T4 | Tetraiodothyronine (thyroxine) |
| TSH | Thyroid-stimulating hormone |
| V | Variable |
| VDRL | Venereal Disease Research Laboratory (syphilis test) |
| WBC | White blood cell |
| Wk | Week |
| Yr | Year |
| ↑ | Increased |
| ↓ | Decreased |
| ↔ | No change |
Diagnostic Testing and Medical Decision Making: Introduction
The clinician’s main task is to make reasoned decisions about patient care despite incomplete clinical information and uncertainty about clinical outcomes. Although data elicited from the history and physical examination are often sufficient for making a diagnosis or for guiding therapy, more information may be required. In these situations, clinicians often turn to diagnostic tests for help.
Benefits, Costs, and Risks
When used appropriately, diagnostic tests can be of great assistance to the clinician. Tests can be helpful for screening, ie, to identify risk factors for disease and to detect occult disease in asymptomatic persons. Identification of risk factors may allow early intervention to prevent disease occurrence, and early detection of occult disease may reduce disease morbidity and mortality through early treatment. Blood pressure measurement is recommended for preventive care of asymptomatic low risk adults. Screening for breast, cervix, and colon cancer is also recommended, whereas screening for prostate cancer and lung cancer remains controversial. Optimal screening tests should meet the criteria listed in Table 1–1.
| Characteristics of population |
| 1. Sufficiently high prevalence of disease. |
| 2. Likely to be compliant with subsequent tests and treatments. |
| Characteristics of disease |
| 1. Significant morbidity and mortality. |
| 2. Effective and acceptable treatment available. |
| 3. Presymptomatic period detectable. |
| 4. Improved outcome from early treatment. |
| Characteristics of test |
| 1. Good sensitivity and specificity. |
| 2. Low cost and risk. |
| 3. Confirmatory test available and practical. |
Tests can also be helpful for diagnosis, ie, to help establish or exclude the presence of disease in symptomatic persons. Some tests assist in early diagnosis after onset of symptoms and signs; others assist in developing a differential diagnosis; others help determine the stage or activity of disease.
Tests can be helpful in patient management: (1) to evaluate the severity of disease, (2) to estimate prognosis, (3) to monitor the course of disease (progression, stability, or resolution), (4) to detect disease recurrence, and (5) to select drugs and adjust therapy.
When ordering diagnostic tests, clinicians should weigh the potential benefits against the potential costs and adverse effects. Some tests carry a risk of morbidity or mortality—eg, cerebral angiogram leads to stroke in 0.5% of cases. The potential discomfort associated with tests such as colonoscopy may deter some patients from completing a diagnostic work-up. The result of a diagnostic test may mandate additional testing or frequent follow-up, and the patient may incur significant cost, risk, and discomfort during follow-up procedures.
Furthermore, a false-positive test may lead to incorrect diagnosis or further unnecessary testing. Classifying a healthy patient as diseased based on a falsely positive diagnostic test can cause psychological distress and may lead to risks from unnecessary or inappropriate therapy. A screening test may identify disease that would not otherwise have been recognized and that would not have affected the patient. For example, early-stage prostate cancer detected by prostate-specific antigen (PSA) screening in a 76-year-old man with known congestive heart failure will probably not become symptomatic during his lifetime, and aggressive treatment may result in net harm.
The costs of diagnostic testing must also be understood and considered. Total costs may be high, or cost-effectiveness may be unfavorable. Even relatively inexpensive tests may have poor cost-effectiveness if they produce very small health benefits.
Factors adversely affecting cost-effectiveness include ordering a panel of tests when one test would suffice, ordering a test more frequently than necessary, and ordering tests for medical record documentation only. The operative question for test ordering is, “Will the test result affect patient management?” If the answer is no, then the test is not justified. Unnecessary tests generate unnecessary labor, reagent, and equipment costs and lead to high health care expenditures.
Molecular and genetic testing is becoming more readily available, but its cost-effectiveness and health outcome benefits need to be carefully examined. Diagnostic genetic testing based on symptoms (eg, testing for fragile X in a boy with mental retardation) differs from predictive genetic testing (eg, evaluating a healthy person with a family history of Huntington disease) and from predisposition genetic testing, which may indicate relative susceptibility to certain conditions or response to certain drug treatment (eg, BRCA-1 or HER-2 testing for breast cancer). The outcome benefits of many new pharmacogenetic tests have not yet been established by prospective clinical studies; eg, there is insufficient evidence that genotypic testing for warfarin dosing leads to outcomes that are superior to those using conventional dosing algorithms, in terms of reduction of out-of-range INRs. Other testing (eg, testing for inherited causes of thrombophilia, such as factor V Leiden, prothrombin mutation, etc) has only limited value for treating patients, since knowing whether a patient has inherited thrombophilia generally does not change the intensity or duration of anticoagulation treatment. Carrier testing (eg, for cystic fibrosis) and prenatal fetal testing (eg, for Down syndrome) often require counseling of patients so that there is adequate understanding of the clinical, social, ethical, and sometimes legal impact of the results.
Clinicians order and interpret large numbers of laboratory tests every day, and the complexity of these tests continues to increase. The large and growing test menu has introduced challenges for clinicians in selecting the correct laboratory test and correctly interpreting the test results. Errors in test selection and test result interpretation are common but often difficult to detect. Using evidence-based testing algorithms that provide guidance for test selection in specific disorders and expert-driven test interpretation (eg, reports and interpretative comments generated by clinical pathologists) can help decrease such errors and improve the timeliness and accuracy of diagnosis.
Performance of Diagnostic Tests
Factors affecting both the patient and the specimen are important. The most crucial element in a properly conducted laboratory test is an appropriate specimen.
Preparation of the patient is important for certain tests—eg, a fasting state is needed for optimal glucose and triglyceride measurements; posture and sodium intake should be strictly controlled when measuring renin and aldosterone levels; and strenuous exercise should be avoided before taking samples for creatine kinase determinations, since vigorous muscle activity can lead to falsely abnormal results.
Careful attention must be paid to patient identification and specimen labeling—eg, two patient identifiers (full name and birth date, or full name and unique institutional identifier, eg, Social Security Number) must be used. Knowing when the specimen was collected may be important. For instance, aminoglycoside levels cannot be interpreted appropriately without knowing whether the specimen was drawn just before (“trough” level) or after (“peak” level) drug administration. Drug levels cannot be interpreted if they are drawn during the drug’s distribution phase (eg, digoxin levels drawn during the first 6 hours after an oral dose). Substances that have a circadian variation (eg, cortisol) can be interpreted only in the context of the time of day the sample was drawn.
During specimen collection, other principles should be remembered. Specimens should not be drawn above an intravenous line, because this may contaminate the sample with intravenous fluid and drug (eg, heparin). Excessive tourniquet time leads to hemoconcentration and an increased concentration of protein-bound substances such as calcium. Lysis of cells during collection of a blood specimen results in spuriously increased serum levels of substances concentrated in cells (eg, lactate dehydrogenase and potassium). Certain test specimens may require special handling or storage (eg, specimens for blood gas and serum cryoglobulin). Delay in delivery of specimens to the laboratory can result in ongoing cellular metabolism and therefore spurious results for some studies (eg, low serum glucose).
Test Characteristics
Table 1–2 lists the general characteristics of useful diagnostic tests. Most of the principles detailed below can be applied not only to laboratory and radiologic tests but also to elements of the history and physical examination. An understanding of these characteristics is very helpful to the clinician when ordering and interpreting diagnostic tests.
| 1. Test methodology has been described in detail so that it can be accurately and reliably reproduced. |
| 2. Test accuracy and precision have been determined. |
| 3. The reference interval has been established appropriately. |
| 4. Sensitivity and specificity have been reliably established by comparison with a gold standard. The evaluation has used a range of patients, including those who have different but commonly confused disorders and those with a spectrum of mild and severe, treated and untreated diseases. The patient selection process has been adequately described so that results will not be generalized inappropriately. |
| 5. Independent contribution to overall performance of a test panel has been confirmed if a test is advocated as part of a panel of tests. |
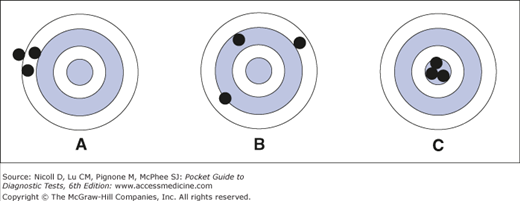
The accuracy of a laboratory test is its correspondence with the true value. A test is deemed inaccurate when the result differs from the true value even though the results may be reproducible (Figure 1–1A), this represents systematic error (or bias). For example, serum creatinine is commonly measured by a kinetic Jaffe method, which has a systematic error as large as 0.23 mg/dL when compared with the gold standard gas chromatography-isotope dilution mass spectrometry method. In the clinical laboratory, accuracy of tests is maximized by calibrating laboratory equipment with reference material and by participation in external proficiency testing programs.
Figure 1–1.
Relationship between accuracy and precision in diagnostic tests. The center of the target represents the true value of the substance being tested. (A) A diagnostic test that is precise but inaccurate; repeated measurements yield very similar results, but all results are far from the true value. (B) A test that is imprecise and inaccurate; repeated measurements yield widely different results, and the results are far from the true value. (C) An ideal test that is both precise and accurate.
Test precision is a measure of a test’s reproducibility when repeated on the same sample. If the same specimen is analyzed many times, some variation in results (random error) is expected; this variability is expressed as a coefficient of variation (CV: the standard deviation divided by the mean, often expressed as a percentage). For example, when the laboratory reports a CV of 5% for serum creatinine and accepts results within ± 2 standard deviations, it denotes that, for a sample with serum creatinine of 1.0 mg/dL, the laboratory may report the result as anywhere from 0.90 to 1.10 mg/dL on repeated measurements from the same sample.
An imprecise test is one that yields widely varying results on repeated measurements (Figure 1–1B). The precision of diagnostic tests, which is monitored in clinical laboratories by using control material, must be good enough to distinguish clinically relevant changes in a patient’s status from the analytic variability (imprecision) of the test. For instance, the manual peripheral white blood cell differential count may not be precise enough to detect important changes in the distribution of cell types, because it is calculated by subjective evaluation of a small sample (eg, 100 cells). Repeated measurements by different technicians on the same sample result in widely differing results. Automated differential counts are more precise because they are obtained from machines that use objective physical characteristics to classify a much larger sample (eg, 10,000 cells).
An ideal test is both precise and accurate (Figure 1–1C).
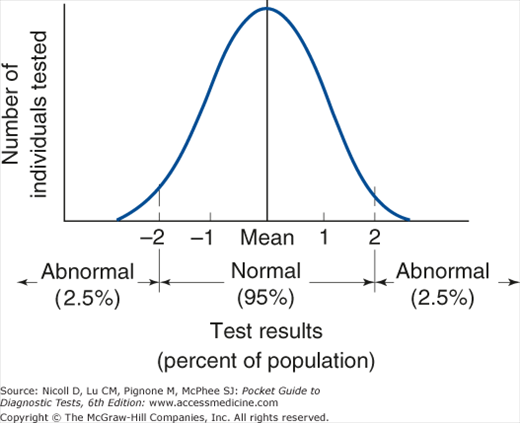
Some diagnostic tests are reported as positive or negative, but many are reported quantitatively. Use of reference intervals is a technique for interpreting quantitative results. Reference intervals are often method- and laboratory-specific. In practice, they often represent test results found in 95% of a small population presumed to be healthy; by definition, then, 5% of healthy patients will have an abnormal test result (Figure 1–2). Slightly abnormal results should be interpreted critically—they may be either truly abnormal or falsely abnormal. Statistically, the probability that a healthy person will have 2 separate test results within the reference interval is (0.95 × 0.95)%, ie, 90.25%; for 5 separate tests, it is 77.4%; for 10 tests, 59.9%; and for 20 tests, 35.8%. The larger the number of tests ordered, the greater the probability that one or more of the test results will fall outside the reference intervals (Table 1–3). Conversely, values within the reference interval may not rule out the actual presence of disease, since the reference interval does not establish the distribution of results in patients with disease.