Tumor Type |
Pattern |
Morphology |
Ancillary Investigations |
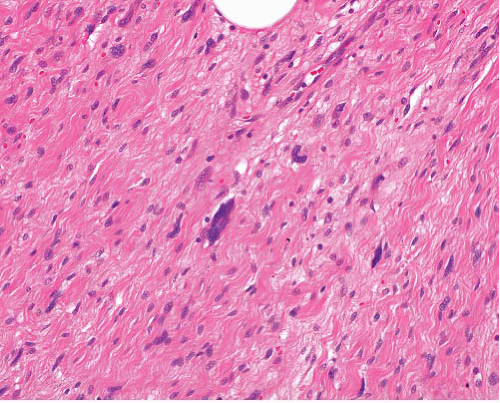
Keloid |
Randomly oriented thick collagen fibers in dermis, can extend beyond site of wound
Low cellularity |
Eosinophilic thick collagen bundles, sparse bland fibroblastic spindle cells |
Nil specific |
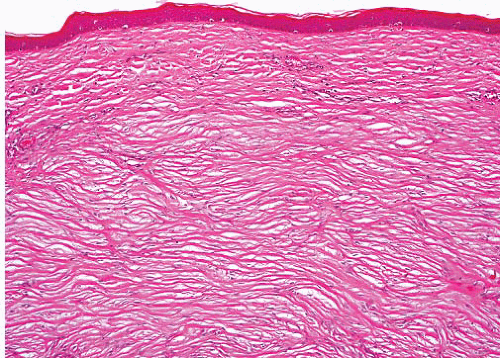
Cellular (hypertrophic) scar |
Variously oriented fibroblasts
More cellular than keloid and restricted to site of wound |
Bland tapered spindle cells, no nuclear atypia |
SMA+, low MIB proliferation index |
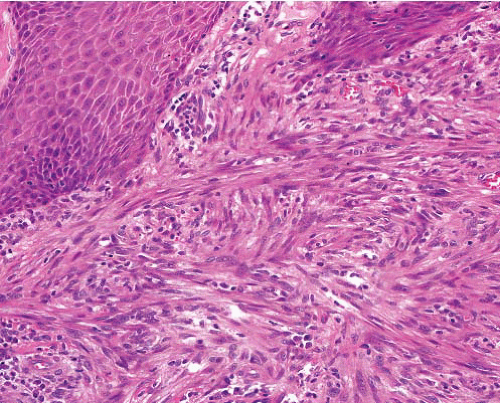
Cellular fibrous histiocytoma |
Epidermal hyperplasia
Cellular fascicles in dermis, extensions into fat, short and pointed, radial to surface
Peripheral collagen bundles |
Uniform tapered cells with ovoid nuclei and scanty cytoplasm, Touton giant cells
Hemorrhage and siderophages if aneurysmal component
Focal cellular pleomorphism in atypical variant |
SMA+, FXIIIa+ |
Dermatofibrosarcoma |
Diffusely storiform, infiltrates parallel to skin surface with extensive honeycombing of fat
Pattern changes to fascicular in fibrosarcomatous transformation. |
Long cells, scanty cytoplasm, uniform tapered, slightly wavy nuclei
Focal or diffuse myxoid change
Mitotic activity increases in fibrosarcomatous variant |
CD34+, bcl-2+. S100 protein + in pigmented cells of Bednar variant
t(17;22)(q22;q13) with fusion gene COL1A1-PDGFRB
Fibrosarcomatous variant similar but can be CD34− |
Giant cell fibroblastoma |
Poorly circumscribed in skin and subcutis, variably cellular without distinct pattern except where coexistent dermatofibrosarcoma
Irregular pseudovascular spaces lined by lesional cells |
Spindle and apparently multinucleated cells (in reality, each has a single highly convoluted nucleus) in fibrous and myxoid stroma |
CD34+, bcl-2+, t(17;22) (q22;q13) with fusion gene COL1A1-PDGFRB |
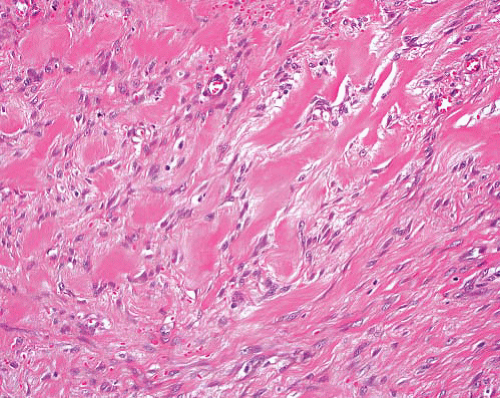
Dermal nerve sheath myxoma |
Circumscribed multilobulated myxoid lesion, low cellularity |
Spindle cells can show focal pleomorphism |
S100 protein+ in myxoid areas, EMA+ at periphery of nodules |
Superficial angiomyxoma |
Noncircumscribed, multinodular, infiltrative in dermis and subcutis
Random pattern, not storiform or fascicular |
Bland short to medium-length spindle or stellate fibroblasts in myxoid stroma, variable vascularity, scattered neutrophils |
CD34+ (focally), S100 protein− |
Neurofibroma, typical |
Nonencapsulated, ill-defined fascicles but not widely infiltrative except diffuse type |
Mixture of wavy cells, axons, collagen fibers, mast cells |
S100 protein+ (not all cells), EMA+ very rarely focally, CD34+ focally. NF+ in axons |
Neurofibroma, diffuse |
Subcutaneous infiltrative plaque
Some associated with plexiform neurofibroma—extends outside nerve bundles into soft tissue |
Sheets of short spindle cells in loose fibrous stroma, infiltrating between normal structures
Wagner-Meissner bodies in various stages |
S100 protein+ diffusely in nuclei |
Atypical fibroxanthoma |
Dome-shaped with overlying epidermal thinning or ulceration
Dermal infiltrate is storiform, fascicular, or patternless |
Pleomorphic spindle and polygonal cells throughout, abnormal mitoses
Clear cell, spindle cell, and giant cell variants |
SMA+ focally, CD10+ |
Kaposi sarcoma |
Cellular, curved fascicles, sievelike areas in cross-section, hemorrhage |
Mildly pleomorphic cells, mitoses, hyaline globules, red cell extravasation |
HHV8+, CD31+, CD34+, podoplanin (D2-40)+
S100 protein− |
Angiosarcoma |
Variable vascular channel formation and cellular areas, with hemorrhage |
Spaces lined by atypical endothelial cells
Solid areas can have spindled or epithelioid cells |
CD34+, CD31+, ERG, FVIIIRAg+, FLI-1+, CK±, S100 protein− protein−, desmin− |
Leiomyoma |
Nodular or diffuse, fascicles intersecting at right angles |
Spindle cells with eosinophilic cytoplasm, nontapering nuclei
No pleomorphism, mitotic activity, or necrosis |
SMA+, desmin+, h-caldesmon+, S100 protein− |
Leiomyosarcoma (and atypical intradermal smooth muscle tumor) |
Fascicles of varying size, intersecting at right angles |
Spindle cells with eosinophilic cytoplasm, nontapering nuclei
Variable pleomorphism, mitotic activity, or necrosis according to grade |
SMA+, desmin+, h-caldesmon+, S100 protein− |
Spindle cell carcinoma |
Sheets, nests, and storiform whorls
Areas of epithelial morphology
Overlying epithelial dysplasia or carcinoma |
Pleomorphic spindle or epithelioid tumor cells
Sheet-like epithelial areas, transitions to sarcomatous morphology
Nested reticulin pattern |
CK+, EMA+, INI1+, CD34 negative, SMA+, P63+ in some, desmin−, h-caldesmon−, S100 protein− |
Myofibroma |
Bundles or whorls of myofibroblastic spindle cells with foci of smaller darker cells, focal pericytomatous pattern
Can be focally necrotic or calcified |
Spindle cells have ovoid nuclei, small nucleoli, tapering eosinophilic cytoplasm
Mitoses in some but no atypia |
SMA+, calponin+, desmin−, h-caldesmon− |
Dermatomyofibroma |
Cellular fascicles run parallel (tangential) to skin surface
Mild adjacent epidermal hyperplasia |
Bland myofibroblastic spindle cells, ovoid nucleoli, small nucleoli, tapering cytoplasm |
SMA+, calponin+, desmin−, h-caldesmon− |
Nodular fasciitis |
Variably myxoid, cellular, and collagenous areas
Mitoses but no nuclear atypia or necrosis |
Spindle cells have ovoid nuclei, small nucleoli, tapering cytoplasm
Mitoses in some but no atypia |
SMA+, desmin±, h-caldesmon− |
Spindle cell lipoma |
Circumscribed, fatty component, collagen fibrils, mast cells
Myxoid variant |
Short spindle cells, very occasional lipoblasts acceptable |
CD34+, rarely S100 protein+, MDM2 and CDK4 usually− |
Perineurioma |
Circumscribed, variably cellular fascicles, with myxoid or fibrous stroma |
Elongated bipolar or tripolar cells with very long terminal processes |
EMA+, claudin-1+, GLUT-1+, CD34+, |
Low-grade fibromyxoid sarcoma |
Ill-defined
Fibrous and myxoid areas with swirling pattern collagen cracking
Occasional hyaline rosettes |
Bland cells with uniformly staining nuclei, sometimes rectangular, indiscernible cytoplasm |
MUC4+, occasional EMA+, claudin-1+. Rarely SMA+ or CD34+
t(7;16)(q34;p11) with fusion gene FUS-CREB3L2 or CREB3L1, rarely t(16;22) (p11;q12) with fusion gene
EWSR1-CREB3L1 |
Malignant melanoma, desmoplastic |
Junctional activity present or absent
Spindle cells singly or in separated bundles infiltrating dermis and subcutis
Neurotropism |
Spindle or focally epithelioid cells with nuclear pleomorphism, scanty cytoplasm, mitoses |
S100 protein+ diffusely, HMB45 or melan-A+ rarely |
Malignant peripheral nerve sheath tumor |
No junctional activity
Bundles and sheaves of spindle cells
Occasional neurotropism |
Elongated spindle cells, wavy or buckled nuclei
Epithelioid areas |
S100 protein+ focally |
Clear cell sarcoma |
Extremities especially lower limb, young adults |
Round or spindled cells in nests, round nuclei with central nucleolus, clear or granular cytoplasm, multinucleated cells, melanin pigment |
S100 protein+, HMB45+ and melan A+ (other markers negative, t(12;22)(q13;q12), EWSR1-ATF1 in soft tissue cases |
PEComa |
Can rarely arise in skin |
Nests of ovoid or spindled cells with abundant clear or rarely granular cytoplasm, delicate fibrous septa |
SMA+, HMB45+, melan-A+, desmin+ in some, TFE3+ in some, CD117+ in some, S100 protein+ rarely |
Anaplastic large cell lymphoma |
Cutaneous involvement can occur with or without nodal disease |
Sheets of cells with prominent nucleoli, multinucleated forms
Can be spindled |
CD30+, ALK+, CD43+, CD45+, CD3+, TIA1+, t(2;5)(p23;q35), TMP3-ALK fusion |
Mycobacterial spindle cell pseudotumor |
Sheets of spindle cells with storiform pattern and plump epithelioid macrophages |
Cells have abundant eosinophilic cytoplasm
Foamy and multinucleated cells sometime seen |
Ziehl-Neelsen stain reveals numerous acid-fast bacilli |