Cutaneous Adverse Reactions to Chemotherapeutic Agents
Grant F. McKinley
Maeve C. Maher
Alejandro A. Gru
Benjamin H. Kaffenberger
REACTIONS TO CHEMOTHERAPEUTIC REGIMENS
Advances in cancer therapy have given rise to a multitude of treatment-related self-limiting or life-threatening mucocutaneous side effects, which can mimic unrelated cutaneous disorders. Knowledge of the clinical presentation, histopathology, and therapy for these reactions is essential in their management, especially when aiming to provide uninterrupted care. A wide variety of chemotherapy drugs are used to target hematologic malignancies. The most common regimens and their mucocutaneous adverse reactions are discussed in this chapter, and they are organized by the disease treated.
ACUTE LEUKEMIAS
Acute leukemia is the most common malignancy in children, making up about 30% of neoplasms in this age group.1 Acute lymphocytic leukemia (ALL) presents about five times more often than its counterpart, acute myeloid leukemia (AML). These malignancies are also well represented in the adolescent age group, making up about 12% of new cancer cases per year,1 although incidence rate of AML tends to increase with age, while ALL peaks in early childhood.2
In the treatment of these disorders, antileukemic therapies target rapidly dividing cells. As a result, other rapidly dividing areas, such as the skin and mucosa, are also commonly affected. This section reviews targeted and cytotoxic therapies by class, commonly used in the induction, consolidation, and maintenance phases of these diseases.
Anthracyclines
Anthracyclines have been widely utilized as antineoplastic agents for systemic malignancy since their isolation from the Streptomyces genus in the 1960s. These drugs work by intercalating between base pairs during DNA replication, ceasing further mitotic activity, as well as producing cytotoxic free oxygen radicals that disrupt cell membranes and other vital structures. In the treatment of ALL and AML, daunorubicin, doxorubicin, and idarubicin are commonly used, especially in the induction and consolidation phases. The polyethylene glycol–coated (pegylated) liposomal form of doxorubicin appears to be associated with a lower incidence of significant side effects, such as cardiotoxicity,3 as it concentrates in tumor tissue.4
Liposomal doxorubicin has increased longevity in the circulation, which enhances its cutaneous deposition and side effects.5,6 Two such reactions include the clinically similar but morphologically and regionally distinct palmar-plantar erythrodysesthesia (PPE) syndrome and malignant intertrigo (Fig. 76-1). These syndromes may represent a host-versus-altered-host reaction, as natural killer (NK) cells and cytotoxic T cells are less susceptible to anthracyclines, and implementation of such regimens may result in an increased number of typically suppressed autoreactive lymphocytes.7 Furthermore, the locations of involvement suggest that the damage may be more prevalent in areas with high sweat duct density (palms and soles) or areas where occluded sweat cannot evaporate efficiently, such as in the skin folds.
 FIGURE 76-1. Malignant intertrigo in the setting of induction chemotherapy using doxorubicin. There is an erythematous maculopapular eruption in the intertriginous areas. |
PPE has an incidence between 20%8 and 29%9 in patients taking liposomal doxorubicin. PPE displays vacuolar necrosis of basal keratinocytes with mild spongiosis and scattered dyskeratotic keratinocytes.10 Intertrigo-like eruptions, on the other hand, are typically associated with bacterial11 or fungal12 infiltrate with neutrophils, as well as an interface dermatitis and dysmaturation of the overlying epidermis. Ischemia of the eccrine coils may trigger squamous syringometaplasia (Fig. 76-2).11,13 The microbial component is often secondary to maceration. Treatment is generally supportive, although application of ice packs have been proven as an efficacious strategy for the treatment or prophylaxis of PPE.14 Short-term corticosteroids with topical corticosteroids15 and pyridoxine supplementation with antioxidant vitamins12,16 have also been useful in reducing the severity of both PPE and intertrigo-like erosions.
 FIGURE 76-2. Malignant intertrigo. A-D. Vacuolar interface changes with dyskeratotic cells, and focal squamous syringometaplasia (400×). |
Doxorubicin is well known for causing a vesicant chemical cellulitis, occurring in 2%17 to 5%18 of patients. This presents initially with erythematous, burning lesions that progress to vesicles about intravenous or other access sites. Histopathology demonstrates epidermal hyperplasia and ulceration with ischemic changes in the epidermis and dermis that may spread to deeper structures. Focal lymphocytic satellitosis may also be present.19 Any areas of necrosis are typically debrided, while rescue therapy includes dexrazoxane.20 Intralesional granulocyte-macrophage colony-stimulating factor (GM-CSF) has also shown potential benefits in recovery after extravasation injuries,20 while cold compresses and corticosteroids may be added to doxorubicin recovery but are contraindicated with vinca alkaloid extravasations.21
Anagen effluvium is another toxicity commonly associated with the anthracyclines, and doxorubicin in particular. It typically occurs about 5 to 30 days after initiation of therapy.17,22,23,24 In animal models, alopecia is preceded by reduction in size of sebocytes with depleted secretory granules. Increased apoptosis of sebocytes leads to apoptosis of connective tissue sheath and matrix cells.22 Also, dose-related stomatitis presents after 1 to 2 weeks of treatment25 in 11% to 70% of cases.18,23,26 Both of these conditions are self-limiting, reversible, and typically treated with supportive therapy. No reduction in dosage is usually required. The patient may also experience acute hypersensitivity reactions, typically type I, which require discontinuation of therapy.27,28
Neutrophilic eccrine hidradenitis (NEH) is another rare side effect of these medications, which may trigger subsequent reduction or cessation of dosing.29,30 NEH is characterized by neutrophilic infiltration around eccrine glands in the dermis leading to necrosis of the eccrine cells (Figs. 76-3 and 76-4).
 FIGURE 76-3. NEH secondary to doxorubicin manifested by a prominent erythematous plaque on the forearm. |
Chromonychia with transverse bands of white and brown coloring due to dysfunction of the nail plate31 is also associated with anthracyclines. Onycholysis with associated pain has also been seen in conjunction with doxorubicin therapy.32,33,34 It appeared to be reversible with cessation of successful chemotherapy,32 and it may correlate with zinc deficiency.33
Radiation recall dermatitis presenting with erythematous desquamation at sites of prior radiotherapy can be also seen in patients receiving doxorubicin. The histopathology often overlaps with acute radiation dermatitis. Other features might include interface reaction (lymphocytic exocytosis, basal vacuolization, apoptotic or necrotic keratinocytes), psoriasiform acanthosis, or follicular hyperkeratosis. Inflammatory cells are typically mixed populations of neutrophils and lymphocytes in a perivascular and interstitial distribution. The dermis might show vascular dilatation and endothelial atypia. The subcutis is typically unaffected.35 Treatment is generally supportive without any need for corticosteroids, and the lesion tends to resolve over a month’s time.35,36
Oral cavity squamous cell carcinomas (SCCs) also seem to be correlated with a history of prolonged pegylated liposomal doxorubicin use, notably in middle-aged patients with no history of alcohol or tobacco exposure.37,38,39,40 This may be secondary to an increased exposure of the mucous membranes to the chemotherapeutic agent because of its increased duration within the vasculature.41 Treatment includes excision and subsequent routine oral examinations.
Hyperpigmentation, generalized or on the digits and nail beds, is also commonly associated with doxorubicin.42 The mechanism seems to be secondary to an undiscovered direct stimulation of melanocytes themselves43 rather than excessive melanocyte-stimulating hormone from the pituitary.44 The nails may appear diffusely discolored with some longitudinal banding, and this abnormal nail pigmentation appears to be more common in the black population.45 There have been reported cases of discoloration of mucosal surfaces during treatment as well.42,46 Histopathologically, these lesions have an increase in melanophages in the lamina propria with increased melanin throughout the mucosal epithelium.42 The hyperpigmentation does not require additional therapy, and it tends to subside after the therapy has been discontinued.
Antimetabolites
Antimetabolites block DNA synthesis as nucleoside analogs. They include cytarabine, a drug used for consolidation therapy in AML and ALL, as well as 6-mercaptopurine (6MP) and methotrexate (MTX), which are often used simultaneously as a maintenance therapy in ALL. MTX is occasionally used for consolidation therapy as well.
Cytarabine
Cytarabine, a purine analog, has cutaneous side effects that are dose-dependent and become more prevalent with an increased duration of therapy.47,48 In one study, 53% of patients treated with high-dose cytarabine demonstrated cutaneous eruptions in response to the therapy, most commonly either a mild acral erythema or transient morbilliform eruption in about 40% of cases.47 It should be noted that moderate-to-severe cases of cytarabine-induced acral erythema may present with a bullous variant.47,49,50,51,52,53 Cytarabine has also had a strong association with NEH.54,54,55,56 NEH and mild acral erythema may be treated with supportive care without any alterations in therapy, as they should resolve spontaneously,55 while severe acral erythema may need a dose reduction for the patient’s benefit.57 Cytarabine may also cause inflamed seborrheic keratoses termed the pseudosign of Leser–Trelat,58,59 as well as hypersensitivity reactions.60 It has also rarely been mentioned with toxic epidermal necrolysis (TEN),61 evident by a necrotic epidermis.
Another important dermatologic side effect of cytarabine therapy is papular purpuric eruption (Fig. 76-5). Thrombotic thrombocytopenic purpura has been reported in connection to intense consolidation therapy for AML. Cytarabine likely damages the vascular endothelium causing blockage with eosinophilic material and swelling of the endothelium62 along with fibrinoid necrosis of the vessel walls with perivascular myxoid degeneration.63 This results in petechiae and sometimes palpable purpura of the skin (Fig. 76-6). Despite the palpable purpura, the papular purpuric eruption is not a leukocytoclastic vasculitis. Treatment of these skin lesions consists of steroids, plasma exchange, and rituximab, but interestingly, future use of cytarabine is not contraindicated. Conversely, cytarabine can also cause palpable purpura in conjunction with other manifestations of Henoch–Schönlein purpura (HSP). The purpura results from vasculitis that histologically shows superficial perivascular neutrophils with prominent leukocytoclasia along with fibrinoid necrosis of the involved venules.64 If these lesions occur, cytarabine will likely have to be discontinued, and the vasculitis can be treated with corticosteroids.
Methotrexate/6-Mercaptopurine
This regimen is most commonly associated with mucositis as a side effect.65,66,67,68 6MP is less commonly associated with acral erythema,69 enhancement of radiation therapy,70 and photo-onycholysis associated with chemotherapy-induced pellagra, which typically presents two or more weeks after initiation of 6MP treatment.71,72
Methotrexate
MTX, due to its role as an irreversible dihydrofolate reductase inhibitor, affects all rapidly proliferating cells, causing an array of clinical side effects in a dose-dependent manner.73 Its toxicity also seems to arise from a long elimination half-life.74 Progressive alopecia75 and mucositis74,76,77 top the list of appreciable cutaneous disorders. An uncommon but significant finding with MTX chemotherapy is reactivation of previous solar erythema,78,79,80 which typically presents 48 hours after receiving MTX.78 The prior solar burns are usually recent, and leucovorin rescue has not been found to ameliorate the issue.80 In patients with recent solar burns, it is recommended that MTX therapy be started about 1 week after the effects subside.78 Its use should be reconsidered if paired concomitantly with either recreational or therapeutic ultraviolet (UV) light exposure.79
MTX has also been associated with oral and cutaneous ulcerations and B-cell lymphoproliferative disorders (LPD) (see Chapter 46 for details on Epstein–Barr virus (EBV)-positive mucocutaneous ulcer and MTX-associated B-cell LPDs).81,82,83 Histologically, sheets of immunoblast-like cells and scattered EBV+ Reed–Sternberg-like cells with CD30 and MUM1 expression is characteristic. EBV-negative cases lack CD30 expression. Other features of Hodgkin lymphoma (HL) are lacking from these cases.82 MTX-induced lymphoproliferations typically clear in 3 weeks after cessation of MTX infusions.83 Close monitoring is suggested after discontinuation, as recurrence of LPDs has been noted in the literature.84
MTX also has some association with PPE,85,86,87 occasionally with a bullous variant,88,89,90,91 as well as horizontal bands of hyperpigmentation that involve the hair in a “flag-like” pattern,92 hypersensitivity reactions,93 and TEN at high doses (Figs. 76-7 and 76-8).94,95,96
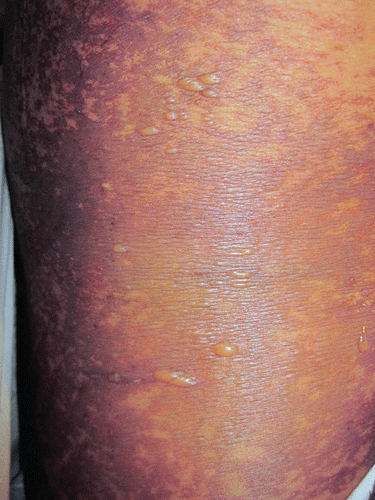 FIGURE 76-7. TEN caused by methotrexate. In this case, there are occasional annular plaques and numerous blisters. |
Enzymes and Enzyme Inhibitors
L-Asparaginase
The Escherichia coli–derived L-asparaginase is a polypeptide enzyme typically used in the induction phase of ALL treatment. It is classically associated with hypersensitivity reactions, typically type I, that occur in 15%97 to 24%98 of patients studied. An urticarial eruption would histologically demonstrate a cellular infiltrate of neutrophils, eosinophils, and leukocytes in the dermis with neutrophil margination within capillaries and prominent papillary dermal edema.99 This hypersensitivity is believed to be induced by the bacterial origins of its polypeptide chain.100 This commonly reported toxicity often requires termination of asparaginase treatment, which has been associated with significantly worse outcomes in patients being treated for ALL.101 Substitution from the native E. coli asparaginase to the Erwinia derivative has provided a reduction in hypersensitivity prevalence, although cross-reactivity has been observed in some patients.97 There is also evidence that pegylated asparaginase maintains the efficacy of the native form, while providing substantial decreases in hypersensitivity reactions owing to its decreased immunogenicity.102 Early detection of a “silent hypersensitivity” may prove crucial in determining the potential for hypersensitivity reaction in a patient, and the need for substitution with an alternative. This can be done by quantifying the serum levels of anti-asparaginase antibody positivity in a given patient, which neutralizes asparaginase activity and predisposes for hypersensitivity reactions.103,104
Etoposide (Topoisomerase II Inhibitor)
Etoposide is a podophyllotoxin derivative used in both solid and hematologic malignancies to bind microtubular proteins and inhibit mitosis. In the treatment of ALL, it is used during the consolidation phase. It has been associated with a generalized erythematous maculopapular rash.105 The characteristic histologic findings include keratinocytes with pale cytoplasm and scattered chromatin within fragmented nuclei, referred to as “starburst cells.” The epidermis examined was otherwise noted to be dyskeratotic. These reactions to therapy are generally self-limiting. They present within 5 to 9 days after etoposide therapy induction and resolve without specific treatment within 3 weeks.105
Another characteristic rash is PPE, which is painful and localized to the palms and soles.10 This eruption consisted of erythematous patches that were mostly focal over the palmar surface, starting about the thenar eminence. Histology and treatment plans were similar to previously described PPE.106
Etoposide also has been linked to cases of UV radiation recall, displaying perivascular mononuclear cell infiltrate without evidence of dyskeratotic keratinocytes. Symptomatic emollient therapy relieved the reactivation.107 Type I hypersensitivity reactions have also shown some correlation to etoposide induction, reportedly as high as 34% in one study, most cases being grade 1 or 2 in severity.108 These are treated with infusion cessation and introduction of diphenhydramine and systemic corticosteroids. Premedication with diphenhydramine, ranitidine, and systemic corticosteroids in patients with prior reactions may serve as appropriate prophylaxis.109
Vincristine (Vinca Alkaloids)
Vincristine, an inhibitor of microtubule formation, arrests the cell at metaphase by binding to the tubulin dimers involved in mitotic spindle formation. It is used widely for various solid and hematologic malignancies, including HL, non-HL (NHL), and ALL, specifically during the induction and maintenance phases of ALL treatment.
Vincristine is associated with an extravasation reaction, either by leakage or direct infiltration. The erosive consequences of this extravasation may present initially as localized areas of erythema and edema that progress to blistering or firm, necrotic lesions. Treatments should be focused on removal of the chemotherapeutic from the extravasated site. Hyaluronidase has proven efficacious through destruction of subcutaneous tissue bonds,110,111 while topical warm applications can also be used to increase vasodilation about the area.110 Cold compresses are contraindicated with vincristine extravasation therapy, a modality used in the treatment of doxorubicin extravasation.21,110
Vincristine has also been rarely associated with NEH when used in combination therapy. Clinically, this may present with a fever and erythematous nodules, along with a classic histologic appearance of neutrophilic infiltration about the eccrine glands in the dermis with associated epithelial necrosis of the eccrine cells.29 Typically, this condition is self-limiting and resolves in the course of several weeks.
All-Trans Retinoic Acid
All-trans retinoic acid (ATRA) with standard chemotherapy has shown marked improvement in the remission of acute promyelocytic leukemia (APL), an uncommon form of AML with a hallmark, balanced t(15;17) translocation. APL’s translocation fuses the retinoic acid receptor-α (RARα) gene on chromosome 17 to the promyelocytic leukemia gene on chromosome 15, which appears to disrupt the integrity and function of the RARα protein112,113,114 with a resultant characteristic abundance of bilobed promyelocytes on blood smear.115 Auer rods may also be seen. ATRA therapy induces differentiation of the promyelocytes into phenotypically mature cells with continuous exposure,116 inducing significant remission rates in APL patients.117
ATRA has been associated with scrotal ulcerations preceded by a fever with leukocytosis, presenting preferentially in the Asian population. The ulcerations tend to arise between 1 and 3 weeks after initiation of ATRA, and they typically develop into black painful eschars.118,119,120,121,122,123 Histopathologic findings demonstrate nonspecific findings of inflammation with erosion of the epidermis and scattered areas of necrosis and fibrosis.119,122,123 This condition appears to be correlated with a rise in granulocyte colony-stimulating factor (G-CSF) and subsequent direct injury to the tissues, as well as an ATRA-induced increased superoxide production.121 Direct excretion in the urine may explain the preference for scrotal involvement. ATRA cessation is not indicated for treatment of these ulcerations, and they tend to resolve without specific therapy. Topical corticosteroids and antibiotic ointments may be implemented in the appropriate patient when an accompanying, superimposed bacterial infection is present.122 Proper hygiene and thick emollient creams to the scrotum should also be considered.
These ulcerations should be distinguished from acute febrile neutrophilic dermatosis, or Sweet syndrome (Figs. 76-9 and 76-10), which may also be induced by ATRA. The classic triad of Sweet syndrome includes tender, erythematous plaques or papular vesicles, fever, and leukocytosis with neutrophilia.124 Histology shows dermal neutrophilic infiltrates and papillary dermal edema without vasculitis.125,126 Exclusion of infectious agents by histopathology and tissue culture is a prerequisite for final diagnosis. The pathogenesis of Sweet syndrome is not well understood, although it is believed to be secondary to exogenous induction of cytokine production with a resultant promotion of neutrophilic activation and invasion.127 Dexamethasone or prednisone therapy results in rapid resolution of lesions.125,126 Repeat ATRA treatments in AML patients with prior reactions does not seem to induce recurrences of Sweet syndrome.128
 FIGURE 76-9. ATRA-induced Sweet syndrome. Dermal inflamed nodules in the neck and the trunk in a patient undergoing induction chemotherapy for APL. |
 FIGURE 76-10. ATRA-induced Sweet syndrome. Diffuse dermal infiltrate composed of neutrophils and leukocytoclastic nuclear debris. Prominent superficial dermal edema is also noted (A-D). |
Cheilitis is one of the more common mucocutaneous toxicities of ATRA treatment, presenting in 73%129 to 93%130 of patients, although it is typically minor in severity and treated symptomatically. One study described the rare progression to gangrenous cheilitis, presenting as painful black eschars about the lips.131 It appears to be secondary to the infiltration of tissue destructive neutrophils, similar to what is seen in scrotal ulcerations. The histologic features exhibit neutrophilic infiltrates with or without vasculitis, as well as a lack of microbial etiology. This condition tends to heal slowly over months and typically does not necessitate cessation of ATRA therapy.131
CHRONIC LEUKEMIAS
Chronic leukemias most commonly present in adulthood. Their incidence increases with age.2 The incidence of chronic lymphocytic leukemia (CLL) among new leukemia cases per year in the United States is higher than that of chronic myeloid leukemia (CML) (30% vs. 11%, respectively).132 Hairy-cell leukemia (HCL) presents as a rare form of chronic leukemia, making up about 2% of leukemias each year. Chronic leukemia has a tendency to affect males more than females with a ratio of nearly 2:1 overall, with HCL in particular affecting approximately four times more males than females.2 The mainstays of therapy currently for CLL include rituximab and fludarabine and tyrosine kinase inhibitors (TKIs) for CML. The dermatologic effects of these drugs and others are discussed later.
Monoclonal Antibodies
Monoclonal antibodies (MAbs) are engineered against cell-surface markers implicated in various malignancies. In the treatment of CLL, MAbs have introduced a wide array of new therapeutics in the treatment of refractory patients. These MAbs in CLL include alemtuzumab, ofatumumab, and rituximab.
Alemtuzumab is a recombinant DNA-encoded humanized immunoglobulin G1 (IgG1) MAb that targets the cell-surface protein CD52, which is highly expressed on normal and abnormal mature lymphocytes, both of T- and B-cell origin, but not on hematopoietic stem cells.133 Both ofatumumab134 and rituximab135 target CD20, which is widely expressed on B lineage cells. These MAbs are typically associated with rash, urticaria, and infusion-related reactions upon initial infusion, typically of grade 1 to 2 severity. These usually subside after the first week of therapy.133,134,136,137,138,139,140
Alemtuzumab has recently been shown to have a better safety profile when administered subcutaneously, while sustaining prior efficacy standards seen in infusion studies.133,136,137,141 This should be started after an initial week of infusion therapy, as subcutaneous alemtuzumab has had a greater rate of injection site skin reactions, as well as a prolonged dose escalation, when administered as the introductory therapy. Areas of erythema up to 30 cm were seen in some patients under this subcutaneous dosing.136 An effective route for lessening the prevalence of infusion-related toxicities is to premedicate the patient with 50 mg of diphenhydramine about 30 minutes before the initial infusion.141
Additionally, the inclusion of alemtuzumab with total body irradiation (TBI) and cyclophosphamide (Cy) in a conditioning dose for the patient with CLL before autologous stem cell transplantation causes a marked increase in the incidence rate of autologous graft-versus-host disease (auto-GVHD).142 Twelve of sixteen patients on this therapy developed unexplained skin rashes, while seven of them were clinically diagnosed with auto-GVHD. Comparatively, 11 patients treated preemptively with TBI/Cy alone had no evidence of GVHD or other mucocutaneous conditions. Five patients with the clinical diagnosis of auto-GVHD were biopsied, which demonstrated apoptotic figures in the epidermal basal layer with vacuolar interface changes and superficial perivascular lymphocytic infiltrates. Thus, the addition of alemtuzumab to TBI/Cy therapy should be evaluated through a risk-versus-benefit analysis, as the depletion of T-regulatory cells may predispose toward auto-GVHD.142
Rituximab has been associated with severe mucocutaneous reactions. It has been reported to induce Stevens–Johnson syndrome (SJS) in some patients.143,144 An association with paraneoplastic pemphigus when used in combination therapy with bendamustine had also been noted.145 Paraneoplastic pemphigus demonstrates suprabasal acantholysis and intercellular and linear basement membrane deposition of IgG and C3 in the epidermis. It should be noted that CLL and NHL are the most commonly associated predisposing conditions for paraneoplastic pemphigus.
Rituximab has some association with serum sickness in the classic type III hypersensitivity pattern approximately 1 to 2 weeks after initial infusion,146,147,148,149 secondary to host IgG antibodies targeted against the murine Fab fragments of rituximab.150 Serum sickness usually presents in a febrile patient complaining of arthralgias and a pruritic purpuric eruption. Laboratory findings include decreased C3 and C4 markers with increased erythrocyte sedimentation rates and C-reactive protein (CRP) levels.151 As the classic triad of symptoms allows for clinical diagnosis, the rash in this condition is rarely biopsied; however, it should reveal a leukocytoclastic vasculitis. Treatment involves intravenous methylprednisolone with favorable outcomes. Mild cases of serum sickness, after resolution of symptoms, have been followed with reintroduction of rituximab without recurrence.152
Purine Analogs
Fludarabine, cladribine, and pentostatin are used in oncology as agents that resemble the compounds adenosine and deoxyadenosine, although their mechanisms for induction of cytotoxicity differ significantly. Notably, these chemotherapeutics show a propensity for reducing the CD4/CD8 ratio153 and increasing the susceptibility to opportunistic infections. Fludarabine is used in the treatment of CLL, while cladribine and pentostatin are frequently used in HCL.
There are multiple reports of fludarabine inducing paraneoplastic pemphigus in CLL patients within weeks of induction therapy, presenting as a maculopapular eruption with extensive oral erosions.154,155,156 One case developed into pemphigus vegetans, confirmed by histopathologic findings of acanthosis and suprabasal acantholysis.154 The mucocutaneous eruptions of paraneoplastic pemphigus show marked improvement after discontinuation of the pulsed fludarabine treatments.155,156
Pentostatin has also been linked to cutaneous reactions, most commonly a minor skin reaction seen in 90% of patients157 that may appear erythematous, papular, or vesiculobullous. Higher-grade skin toxicities, including those resulting in termination of therapeutic regimen, and both low- and high-grade stomatitis have also been frequently observed.158,159 Severe, biopsied reactions appear rare, although it should be noted that a clinically diagnosed fatal erythroderma has been attributed to pentostatin injection.160
Some authors have also suggested elevated surveillance when administering purine analogs. Fludarabine161 and cladribine162 when given with nonirradiated donor red blood cells and platelets have been implicated in the development of transfusion-associated GVHD. These two medications are also believed to facilitate aggressive epithelial growth, a side effect likely secondary to the lymphocytic depletion. Accelerated SCC infiltration has been noted, and rapid recurrence and metastasis have been seen in these cases.163,164,165
Tyrosine Kinase Inhibitors
TKIs target the tyrosine kinase receptor, which functions to phosphorylate various downstream signaling proteins involved in malignant transformation. They have become a mainstay of therapy in the treatment of BCR-ABL tyrosine kinase protein–positive diseases. Imatinib is used as a first-line therapy against the BCR-ABL receptor, whereas nilotinib and dasatinib are used in refractory or resistant disease. Ibrutinib is also actively used against the Bruton tyrosine kinase (BTK) receptor in CLL.
Imatinib is an inhibitor of the BCR-ABL receptor, and thus used as a first-line drug in the treatment of Philadelphia chromosome–positive (Ph+) CML and Ph+ ALL. It has been observed that between 10% and 67%166,167,168 of patients will experience a cutaneous adverse event while receiving this medication, the majority of which are dose-dependent,169 mild,166 and self-limiting.168 These typically include edema, particularly localized to the eyelids, and skin rashes, which have been estimated to occur 60% and 32% of the time, respectively.170 Other mild reactions have included photosensitization, believed to be secondary to cumulative dosing of the medication,171 as well as hypopigmentation,172,173 histopathologically showing scant melanin pigmentation secondary to inadequate melanocytes at the basal layer.174
Imatinib has also been implicated in the onset of pseudoporphyria, presenting clinically with hemorrhagic bullae and erosions most commonly on the dorsal and lateral aspects of the hands. A biopsy of the area demonstrated subepidermal vesicles and bullae, with minimal cellular inflammation.175,176 Therapy may be continued despite the presence of these bullae, although clearance generally occurs concomitantly with cessation of imatinib.176 Prevention of trauma is needed if therapy is continued. Imatinib has also been associated with exacerbations of underlying psoriasis, which may become refractory to topical corticosteroids. Cessation of the therapy was required.177
Although most of these reactions are mild to moderate and self-limiting, imatinib has been associated with more severe cutaneous eruptions including Sweet syndrome. Clearance of the plaques can be achieved with discontinuation of imatinib.178 Other severe reactions include erythema multiforme (EM)179 and SJS/TEN.180,181,182 Imatinib can also cause acute generalized pustulosis, clinically presenting with a febrile rash with pustules and papules located diffusely, albeit sparing the palms and soles. Biopsies demonstrate subcorneal pustules with accompanying parakeratosis and an abundance of neutrophils.183 Control of severe, recurrent eruptions may be viably achieved through once-weekly dosing or providing predose and adjuvant administrations of corticosteroids with imatinib.184
Dasatinib and nilotinib are structurally similar to imatinib, and serve as alternates in imatinib-refractory CML. They have generally been recorded with fewer side effects than imatinib, although less reported data may be a contributing factor.169 The vast majority of cutaneous reactions reported tend to be grade 1 or 2 rashes, pruritus, and xeroderma.169,185 Rarer but more severe side effects have also been reported. Nilotinib has been found to induce bullous Sweet syndrome, demonstrating subepidermal edema owing to extreme papillary dermal edema and infiltration by neutrophils and atypical CD68+ monocytoid cells. Bullous lesions disappeared with prednisolone and antibiotics, and nilotinib therapy was continued without recurrence.186 Dasatinib, in turn, has been linked to two cases of lobular panniculitis during CML therapy. These cases presented with painful nodules with overlying erythema, which were microscopically shown to have massive subcutaneous infiltration by neutrophils. These lesions resolved with discontinuation of dasanitib.187
Ibrutinib, a BTK inhibitor (BTKi), plays a key role in neutralizing the constitutive B-cell receptor (BCR) signaling seen in CLL. This medication has been largely efficacious with little toxicity.188,189 Cutaneous and mucocutaneous eruptions are seldom seen with only 8% of patients present with a rash during their course, and 11% demonstrate stomatitis believed to be therapy-related. Conversely, petechiae attributed to ibrutinib use does seem to be more substantial than other medications used for CLL, as it presents in about 14% of cases.189 The main cutaneous side effect other than petechiae is a mixed septal and lobular panniculitis, which can be accompanied by neutrophilia, lymphocytosis, leukocytoclasis, and granulomatous features (Figs. 76-11 and 76-12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






