KEY POINTS
Resection principles: The mesenteric clearance technique dictates the extent of resection and is determined by the nature of the primary pathology, the intent of resection, the location of the lesion, and the condition of the mesentery.
Function after resection: Bowel function is often compromised after colorectal resection, especially after low anterior resection. For this reason, it is important to obtain a history of prior anorectal trauma and/or incontinence before considering a low anastomosis.
Ostomies: Preoperative marking for a planned stoma is critical for a patient’s quality of life. Ideally, a stoma should be located within the rectus muscle, in a location where the patient can easily see and manipulate the appliance, and away from previous scars, bony prominences, or abdominal creases.
Inflammatory bowel disease: Both Crohn’s disease and ulcerative colitis are associated with an increased risk of colorectal carcinoma. Risk depends on the amount of colon involved and the duration of disease.
Pathogenesis of colorectal cancer: A variety of mutations have been identified in colorectal cancer. Mutations may cause activation of oncogenes (K-ras) and/or inactivation of tumor suppressor genes (adenomatous polyposis coli [APC], deleted in colorectal carcinoma [DCC], p53).
Minimally invasive resection: Laparoscopy and HAL have been shown to be both safe and efficacious for colorectal resection.
Anal epidermoid carcinoma: Unlike rectal adenocarcinoma, anal epidermoid carcinoma is treated primarily with chemoradiation. Surgery is reserved for patients with persistent or recurrent disease.
Rectal prolapse: Rectal prolapse occurs most commonly in elderly women. Transabdominal repair (rectopexy with or without resection) offers more durability than perineal proctosigmoidectomy, but carries greater operative risk.
Hemorrhoids: Hemorrhoids are cushions of submucosal tissue containing venules, arterioles, and smooth muscle fiber. They are thought to play a role in maintaining continence. Resection is only indicated for refractory symptoms.
Fistula in ano: Treatment of fistula in ano depends on the location of the fistula, amount of anal sphincter involved in the fistula, and the underlying disease process.
EMBRYOLOGY AND ANATOMY
The embryonic gastrointestinal tract begins developing during the fourth week of gestation. The primitive gut is derived from the endoderm and divided into three segments: foregut, midgut, and hindgut. Both midgut and hindgut contribute to the colon, rectum, and anus.
The midgut develops into the small intestine, ascending colon, and proximal transverse colon, and receives blood supply from the superior mesenteric artery. During the sixth week of gestation, the midgut herniates out of the abdominal cavity, and then rotates 270° counterclockwise around the superior mesenteric artery to return to its final position inside the abdominal cavity during the tenth week of gestation. The hindgut develops into the distal transverse colon, descending colon, rectum, and proximal anus, all of which receive their blood supply from the inferior mesenteric artery. During the sixth week of gestation, the distal-most end of the hindgut, the cloaca, is divided by the urorectal septum into the urogenital sinus and the rectum.
The distal anal canal is derived from ectoderm and receives its blood supply from the internal pudendal artery. The dentate line divides the endodermal hindgut from the ectodermal distal anal canal.
The large intestine extends from the ileocecal valve to the anus. It is divided anatomically and functionally into the colon, rectum, and anal canal. The wall of the colon and rectum comprise five distinct layers: mucosa, submucosa, inner circular muscle, outer longitudinal muscle, and serosa. In the colon, the outer longitudinal muscle is separated into three teniae coli, which converge proximally at the appendix and distally at the rectum, where the outer longitudinal muscle layer is circumferential. In the distal rectum, the inner smooth muscle layer coalesces to form the internal anal sphincter. The intraperitoneal colon and proximal one-third of the rectum are covered by serosa; the mid and lower rectum lack serosa.
The colon begins at the junction of the terminal ileum and cecum and extends 3 to 5 feet to the rectum. The rectosigmoid junction is found at approximately the level of the sacral promontory and is arbitrarily described as the point at which the three teniae coli coalesce to form the outer longitudinal smooth muscle layer of the rectum. The cecum is the widest diameter portion of the colon (normally 7.5–8.5 cm) and has the thinnest muscular wall. As a result, the cecum is most vulnerable to perforation and least vulnerable to obstruction. The ascending colon is usually fixed to the retroperitoneum. The hepatic flexure marks the transition to the transverse colon. The intraperitoneal transverse colon is relatively mobile, but is tethered by the gastrocolic ligament and colonic mesentery. The greater omentum is attached to the anterior/superior edge of the transverse colon. These attachments explain the characteristic triangular appearance of the transverse colon observed during colonoscopy. The splenic flexure marks the transition from the transverse colon to the descending colon. The attachments between the splenic flexure and the spleen (the lienocolic ligament) can be short and dense, making mobilization of this flexure during colectomy challenging. The descending colon is relatively fixed to the retroperitoneum. The sigmoid colon is the narrowest part of the large intestine and is extremely mobile. Although the sigmoid colon is usually located in the left lower quadrant, redundancy and mobility can result in a portion of the sigmoid colon residing in the right lower quadrant. This mobility explains why volvulus is most common in the sigmoid colon and why diseases affecting the sigmoid colon, such as diverticulitis, may occasionally present as right-sided abdominal pain. The narrow caliber of the sigmoid colon makes this segment of the large intestine the most vulnerable to obstruction.
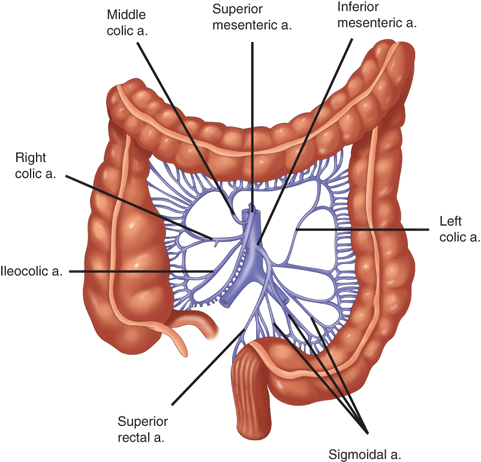
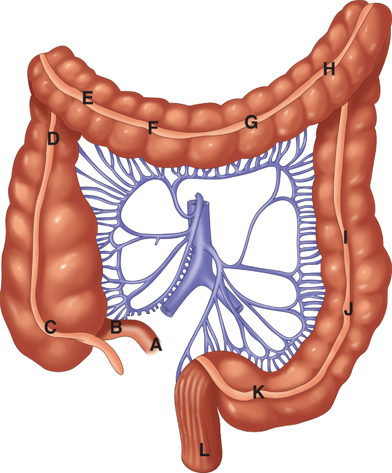
The arterial supply to the colon is highly variable (Fig. 29-1). In general, the superior mesenteric artery branches into the ileocolic artery (absent in up to 20% of people), which supplies blood flow to the terminal ileum and proximal ascending colon; the right colic artery, which supplies the ascending colon; and the middle colic artery, which supplies the transverse colon. The inferior mesenteric artery branches into the left colic artery, which supplies the descending colon; several sigmoidal branches, which supply the sigmoid colon; and the superior rectal artery, which supplies the proximal rectum. The terminal branches of each artery form anastomoses with the terminal branches of the adjacent artery and communicate via the marginal artery of Drummond. This arcade is complete in only 15% to 20% of people.
Except for the inferior mesenteric vein, the veins of the colon parallel their corresponding arteries and bear the same terminology (Fig. 29-2). The inferior mesenteric vein ascends in the retroperitoneal plane over the psoas muscle and continues posterior to the pancreas to join the splenic vein. During a colectomy, this vein is often mobilized independently and ligated at the inferior edge of the pancreas.
The lymphatic drainage of the colon originates in a network of lymphatics in the muscularis mucosa. Lymphatic vessels and lymph nodes follow the regional arteries. Lymph nodes are found on the bowel wall (epicolic), along the inner margin of the bowel adjacent to the arterial arcades (paracolic), around the named mesenteric vessels (intermediate), and at the origin of the superior and inferior mesenteric arteries (main). The sentinel lymph nodes are the first one to four lymph nodes to drain a specific segment of the colon and are thought to be the first site of metastasis in colon cancer. The utility of sentinel lymph node dissection and analysis in colon cancer remains controversial.
The colon is innervated by both sympathetic (inhibitory) and parasympathetic (stimulatory) nerves, which parallel the course of the arteries. Sympathetic nerves arise from T6-T12 and L1-L3. The parasympathetic innervation to the right and transverse colon is from the vagus nerve; the parasympathetic nerves to the left colon arise from sacral nerves S2-S4 to form the nervi erigentes.
The rectum is approximately 12 to 15 cm in length. Three distinct submucosal folds, the valves of Houston, extend into the rectal lumen. Posteriorly, the presecral fascia separates the rectum from the presacral venous plexus and the pelvic nerves. At S4, the rectosacral fascia (Waldeyer’s fascia) extends forward and downward and attaches to the fascia propria at the anorectal junction. Anteriorly, Denonvilliers’ fascia separates the rectum from the prostate and seminal vesicles in men and from the vagina in women. The lateral ligaments support the lower rectum.
The anatomic anal canal extends from the dentate or pectinate line to the anal verge. The dentate or pectinate line marks the transition point between columnar rectal mucosa and squamous anoderm. The anal transition zone includes mucosa proximal to the dentate line that shares histologic characteristics of columnar, cuboidal, and squamous epithelium. Although the anal transition zone was long thought to extend only 1 to 2 cm proximal to the dentate line, it is known that the proximal extent of this zone is highly variable and can be as far as 15 cm proximal to the dentate line. The dentate line is surrounded by longitudinal mucosal folds, known as the columns of Morgagni, into which the anal crypts empty. These crypts are the source of cryptoglandular abscesses (Fig. 29-3). In contrast to the anatomic anal canal, the surgical anal canal begins at the anorectal junction and terminates at the anal verge. The surgical anal canal measures 2 to 4 cm in length and is generally longer in men than in women. It begins at the anorectal junction and terminates at the anal verge.
In the distal rectum, the inner smooth muscle is thickened and comprises the internal anal sphincter that is surrounded by the subcutaneous, superficial, and deep external sphincter. The deep external anal sphincter is an extension of the puborectalis muscle. The puborectalis, iliococcygeus, and pubococcygeus muscles form the levator ani muscle of the pelvic floor (Fig. 29-4).
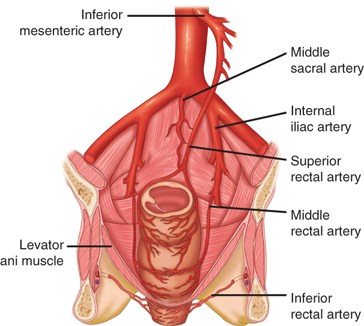
The superior rectal artery arises from the terminal branch of the inferior mesenteric artery and supplies the upper rectum. The middle rectal artery arises from the internal iliac; the presence and size of these arteries are highly variable. The inferior rectal artery arises from the internal pudendal artery, which is a branch of the internal iliac artery. A rich network of collaterals connects the terminal arterioles of each of these arteries, thus making the rectum relatively resistant to ischemia (Fig. 29-5).
The venous drainage of the rectum parallels the arterial supply. The superior rectal vein drains into the portal system via the inferior mesenteric vein. The middle rectal vein drains into the internal iliac vein. The inferior rectal vein drains into the internal pudendal vein, and subsequently into the internal iliac vein. A submucosal plexus deep to the columns of Morgagni forms the hemorrhoidal plexus and drains into all three veins.
Lymphatic drainage of the rectum parallels the vascular supply. Lymphatic channels in the upper and middle rectum drain superiorly into the inferior mesenteric lymph nodes. Lymphatic channels in the lower rectum drain both superiorly into the inferior mesenteric lymph nodes and laterally into the internal iliac lymph nodes. The anal canal has a more complex pattern of lymphatic drainage. Proximal to the dentate line, lymph drains into both the inferior mesenteric lymph nodes and the internal iliac lymph nodes. Distal to the dentate line, lymph primarily drains into the inguinal lymph nodes, but can also drain into the inferior mesenteric lymph nodes and internal iliac lymph nodes.
Both sympathetic and parasympathetic nerves innervate the anorectum. Sympathetic nerve fibers are derived from L1-L3 and join the preaortic plexus. The preaortic nerve fibers then extend below the aorta to form the hypogastric plexus, which subsequently joins the parasympathetic fibers to form the pelvic plexus. Parasympathetic nerve fibers are known as the nervi erigentes and originate from S2-S4. These fibers join the sympathetic fibers to form the pelvic plexus. Sympathetic and parasympathetic fibers then supply the anorectum and adjacent urogenital organs.
The internal anal sphincter is innervated by sympathetic and parasympathetic nerve fibers; both types of fibers inhibit sphincter contraction. The external anal sphincter and puborectalis muscles are innervated by the inferior rectal branch of the internal pudendal nerve. The levator ani receives innervation from both the internal pudendal nerve and direct branches of S3 to S5. Sensory innervation to the anal canal is provided by the inferior rectal branch of the pudendal nerve. While the rectum is relatively insensate, the anal canal below the dentate line is sensate.
Perturbation of the embryologic development of the midgut and hindgut may result in anatomic abnormalities of the colon, rectum, and anus. Failure of the midgut to rotate and return to the abdominal cavity during the tenth week of gestation results in varying degrees of intestinal malrotation and colonic nonfixation. Failure of canalization of the primitive gut can result in colonic duplication. Incomplete descent of the urogenital septum may result in imperforate anus and associated fistulas to the genitourinary tract. Many infants with congenital anomalies of the hindgut have associated abnormalities in the genitourinary tract.
NORMAL PHYSIOLOGY
The colon is a major site for water absorption and electrolyte exchange. Under normal circumstances, approximately 90% of the water contained in ileal fluid is absorbed in the colon (1000–2000 mL/d), but up to 5000 mL of fluid can be absorbed daily. Sodium is absorbed actively via sodium-potassium (Na+/K+) ATPase. The colon can absorb up to 400 mEq of sodium per day. Water accompanies the transported sodium and is absorbed passively along an osmotic gradient. Potassium is actively secreted into the colonic lumen and absorbed by passive diffusion. Chloride is absorbed actively via a chloride-bicarbonate exchange.
Bacterial degradation of protein and urea produces ammonia. Ammonia is subsequently absorbed and transported to the liver. Absorption of ammonia depends in part on intraluminal pH. A decrease in colonic bacteria (e.g., due to broad-spectrum antibiotic use) and/or a decrease in intraluminal pH (e.g., due to lactulose administration) will decrease ammonia absorption.
Short-chain fatty acids (acetate, butyrate, and propionate) are produced by bacterial fermentation of dietary carbohydrates. Short-chain fatty acids are an important source of energy for the colonic mucosa, and metabolism by colonocytes provides energy for processes such as active transport of sodium. Lack of a dietary source for production of short-chain fatty acids, or diversion of the fecal stream by an ileostomy or colostomy, may result in mucosal atrophy and inflammation, the latter termed “diversion colitis.”
Approximately 30% of fecal dry weight is composed of bacteria (1011–1012 bacteria/g of feces). Anaerobes are the predominant class of microorganism, and Bacteroides species are the most common (1011–1012 organisms/mL). Escherichia coli are the most numerous aerobes (108–1010 organisms/mL). Endogenous microflora are crucial for the breakdown of carbohydrates and proteins in the colon and participate in the metabolism of bilirubin, bile acids, estrogen, and cholesterol. Colonic bacteria also are necessary for production of vitamin K. Endogenous bacteria also are thought to suppress the emergence of pathogenic microorganisms, such as Clostridium difficile, a phenomenon termed “colonization resistance.” However, the high bacterial load of the large intestine may contribute to sepsis in critically ill patients and may contribute to intra-abdominal sepsis, abscess, and wound infection following colectomy.
Intestinal gas arises from swallowed air, diffusion from the blood, and intraluminal production. Nitrogen, oxygen, carbon dioxide, hydrogen, and methane are the major components of intestinal gas. Nitrogen and oxygen are largely derived from swallowed air. Carbon dioxide is produced by the reaction of bicarbonate and hydrogen ions and by the digestion of triglycerides to fatty acids. Hydrogen and methane are produced by colonic bacteria. The production of methane is highly variable. The gastrointestinal tract usually contains between 100 and 200 mL of gas, and 400 to 1200 mL/d are released as flatus, depending on the type of food ingested.
Unlike the small intestine, the large intestine does not demonstrate cyclic motor activity characteristic of the migratory motor complex. Instead, the colon displays intermittent contractions of either low or high amplitude. Low-amplitude, short-duration contractions occur in bursts and appear to move the colonic contents both antegrade and retrograde. It is thought that these bursts of motor activity delay colonic transit and thus increase the time available for absorption of water and exchange of electrolytes. High-amplitude contractions occur in a more coordinated fashion and create “mass movements.” Bursts of “rectal motor complexes” also have been described. In general, cholinergic activation increases colonic motility.
Defecation is a complex, coordinated mechanism involving colonic mass movement, increased intra-abdominal and rectal pressure, and relaxation of the pelvic floor. Distention of the rectum causes a reflex relaxation of the internal anal sphincter (the rectoanal inhibitory reflex) that allows the contents to make contact with the anal canal. This “sampling reflex” allows the sensory epithelium to distinguish solid stool from liquid stool and gas. If defecation does not occur, the rectum relaxes and the urge to defecate passes (accommodation response). Defecation proceeds by coordination of increasing intra-abdominal pressure via the Valsalva maneuver, increased rectal contraction, relaxation of the puborectalis muscle, and opening of the anal canal.
The maintenance of fecal continence is at least as complex as the mechanism of defecation. Continence requires adequate rectal wall compliance to accommodate the fecal bolus, appropriate neurogenic control of the pelvic floor and sphincter mechanism, and functional internal and external sphincter muscles. At rest, the puborectalis muscle creates a “sling” around the distal rectum, forming a relatively acute angle that distributes intra-abdominal forces onto the pelvic floor. With defecation, this angle straightens, allowing downward force to be applied along the axis of the rectum and anal canal. The internal and external sphincters are tonically active at rest. The internal sphincter is responsible for most of the resting, involuntary sphincter tone (resting pressure). The external sphincter is responsible for most of the voluntary sphincter tone (squeeze pressure). Branches of the pudendal nerve innervate both the internal and external sphincter. Finally, the hemorrhoidal cushions may contribute to continence by mechanically blocking the anal canal. Thus, impaired continence may result from poor rectal compliance, injury to the internal and/or external sphincter or puborectalis, or nerve damage or neuropathy.
CLINICAL EVALUATION
Obtaining a complete history and performing a physical examination are the starting points for evaluating any patient with suspected disease of the colon, rectum, or anus. Special attention should be paid to the patient’s past medical and surgical history to detect underlying conditions that might contribute to a gastrointestinal problem. If patients have had prior intestinal surgery, it is essential that one understand the resultant gastrointestinal anatomy. A history of anorectal surgery may be critical for patients with either abdominal or anorectal complaints. The obstetrical history in women is essential to detect occult pelvic floor and/or anal sphincter damage. Identifying a family history of colorectal disease, especially inflammatory bowel disease, polyps, and colorectal cancer, is crucial. In addition to a family history of colorectal disease, a history of other malignancies may suggest the presence of a genetic syndrome. Medication use must be detailed as many drugs cause gastrointestinal symptoms. Before recommending operative intervention, the adequacy of medical treatment must be ascertained. In addition to examining the abdomen, visual inspection of the anus and perineum and careful digital rectal exam are essential.
The anoscope is a useful instrument for examination of the anal canal. Anoscopes are made in a variety of sizes and measure approximately 8 cm in length. A larger anoscope provides better exposure for anal procedures such as rubber band ligation or sclerotherapy of hemorrhoids. The anoscope, with obturator in place, should be adequately lubricated and gently inserted into the anal canal. The obturator is withdrawn, inspection of the visualized anal canal is done, and the anoscope should then be withdrawn. It is rotated 90° and reinserted to allow visualization of all four quadrants of the canal. If the patient complains of severe perianal pain and cannot tolerate a digital rectal examination, anoscopy should not be attempted without anesthesia.
The rigid proctoscope is useful for examination of the rectum and distal sigmoid colon and is occasionally used therapeutically. The standard proctoscope is 25 cm in length and available in various diameters. Most often, a 15- or 19-mm diameter proctoscope is used for diagnostic examinations. The large (25-mm diameter) proctoscope is useful for procedures such as polypectomy, electrocoagulation, or detorsion of a sigmoid volvulus. A smaller “pediatric” proctoscope (11-mm diameter) is better tolerated by patients with anal stricture. Suction is necessary for an adequate proctoscopic examination.
Video or fiberoptic flexible sigmoidoscopy and colonoscopy provide excellent visualization of the colon and rectum. Sigmoidoscopes measure 60 cm in length. Full depth of insertion may allow visualization as high as the splenic flexure, although the mobility and redundancy of the sigmoid colon often limit the extent of the examination. Partial preparation with enemas is usually adequate for sigmoidoscopy, and most patients can tolerate this procedure without sedation. Colonoscopes measure 100 to 160 cm in length and are capable of examining the entire colon and terminal ileum. A complete oral bowel preparation is usually necessary for colonoscopy, and the duration and discomfort of the procedure usually require conscious sedation. Both sigmoidoscopy and colonoscopy can be used diagnostically and therapeutically. Electrocautery should generally not be used in the absence of a complete bowel preparation because of the risk of explosion of intestinal methane or hydrogen gases. Diagnostic colonoscopes possess a single channel through which instruments such as snares, biopsy forceps, or electrocautery can be passed; this channel also provides suction and irrigation capability. Therapeutic colonoscopes possess two channels to allow simultaneous suction/irrigation and the use of snares, biopsy forceps, or electrocautery.
Capsule endoscopy is an emerging technology that uses a small ingestible camera. After swallowing the camera, images of the mucosa of the gastrointestinal tract are captured, transmitted by radiofrequency to a belt-held receiver, and then downloaded to a computer for viewing and analysis. Capsule endoscopy largely has been used to detect small bowel lesions. However, it has been suggested that this technique might also be useful for diagnosing colorectal disease, although utility in the evaluation of colorectal disease remains unproven.1,2,3,4,5 Finally, recent advances in the development of maneuverable capsules may improve the sensitivity of this procedure.5
Despite advanced radiologic techniques, plain X-rays and contrast studies continue to play an important role in the evaluation of patients with suspected colon and rectal diseases. Plain X-rays of the abdomen (supine, upright, and diaphragmatic views) are useful for detecting free intra-abdominal air, bowel gas patterns suggestive of small or large bowel obstruction, and volvulus. Contrast studies are useful for evaluating obstructive symptoms, delineating fistulous tracts, and diagnosing small perforations or anastomotic leaks. Although Gastrografin cannot provide the mucosal detail provided by barium, this water-soluble contrast agent is recommended if perforation or leak is suspected. Double-contrast barium enema (use of barium followed by the insufflation of air into the colon) has been reported to be 70% to 90% sensitive for the detection of mass lesions greater than 1 cm in diameter.6 Detection of small lesions can be extremely difficult, especially in a patient with extensive diverticulosis. For this reason, a colonoscopy is preferred for evaluating nonobstructing mass lesions in the colon. Double-contrast barium enema has been used as a back-up examination if colonoscopy is incomplete.
Computed tomography (CT) commonly is employed in the evaluation of patients with abdominal complaints. Its utility is primarily in the detection of extraluminal disease, such as intra-abdominal abscesses and pericolic inflammation, and in staging colorectal carcinoma, because of its sensitivity in detection of hepatic metastases.7
Extravasation of oral or rectal contrast may also confirm the diagnosis of perforation or anastomotic leak. Nonspecific findings such as bowel wall thickening or mesenteric stranding may suggest inflammatory bowel disease, enteritis/colitis, or ischemia. A standard CT scan is relatively insensitive for the detection of intraluminal lesions; however, improved CT technology is increasing the ability to assess bulky rectal tumors and perirectal adenopathy.
CT colonography (virtual colonoscopy) is a radiologic technique that is designed to overcome some of the limitations of traditional CT scanning. This technology uses helical CT and three-dimensional reconstruction to detect intraluminal colonic lesions. Oral bowel preparation, oral and rectal contrast, and colon insufflation have been used to maximize sensitivity. Experience with this technology has shown a sensitivity and specificity for detecting 1 cm or larger polyps of 85% to 90% in most studies, making it comparable to traditional colonoscopy. The addition of “fecal tagging” agents that create a distinct difference in appearance between stool and mucosa during imaging studies holds promise for increasing accuracy and possibly decreasing the need for mechanical bowel preparation.8
The main use of magnetic resonance imaging (MRI) in colorectal disorders is in evaluation of pelvic lesions. MRI is more sensitive than CT for detecting bony involvement or pelvic sidewall extension of rectal tumors. MRI accurately determines the extent of spread of rectal cancer into the adjacent mesorectum and can reliably predict difficulty achieving radial margin clearance of a rectal cancer by surgery alone. When the radial margin is threatened, neoadjuvant chemoradiation is generally indicated. MRI also can be helpful in the detection and delineation of complex fistulas in ano. The use of an endorectal coil may increase sensitivity.
Positron emission tomography (PET) is used for imaging tissues with high levels of anaerobic glycolysis, such as malignant tumors.6 18F-fluorodeoxyglucose (FDG) is injected as a tracer; metabolism of this molecule then results in positron emission. PET has been used as an adjunct to CT in the staging of colorectal cancer and may prove useful in discriminating recurrent cancer from fibrosis. By combining PET and CT technology (PET/CT), anatomic correlation between regions of high isotope accumulation (“hot spots”) on PET and abnormalities on CT can be determined. PET/CT increasingly is used to diagnose recurrent and/or metastatic colorectal cancer. However, the efficacy and utility of this technology remains unproven.
Angiography is occasionally used for the detection of bleeding within the colon or small bowel. To visualize hemorrhage angiographically, bleeding must be relatively brisk (approximately 0.5 to 1.0 mL per minute). If extravasation of contrast is identified, infusion of vasopressin or angiographic embolization can be therapeutic. If surgical resection is required, the angiographic catheter can be left in place to assist with identification of the bleeding site intraoperatively.
CT and magnetic resonance angiography are also useful for assessing patency of visceral vessels. This technique uses three-dimensional reconstruction to detect vascular lesions. If an abnormality is found, more traditional techniques (angiography, surgery) may then be used to further define and/or correct the problem.
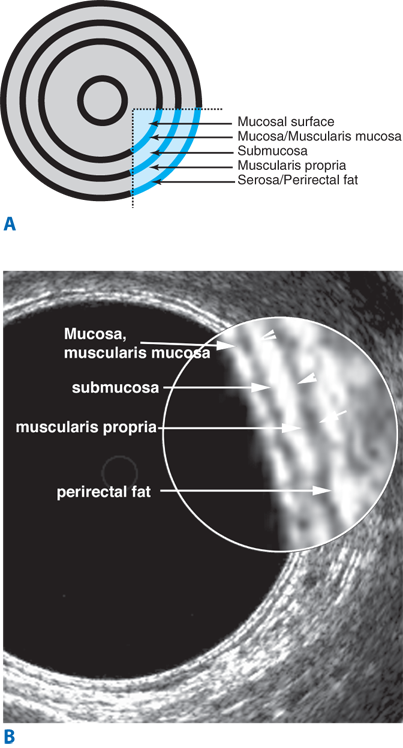
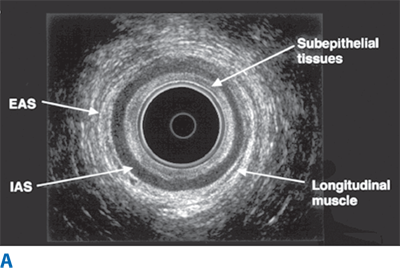
Endorectal ultrasound is primarily used to evaluate the depth of invasion of neoplastic lesions in the rectum. The normal rectal wall appears as a five-layer structure (Fig. 29-6). Ultrasound can reliably differentiate most benign polyps from invasive tumors based on the integrity of the submucosal layer. Ultrasound can also differentiate superficial T1-T2 from deeper T3-T4 tumors. Overall, the accuracy of ultrasound in detecting depth of mural invasion ranges between 81% and 94%.9 This modality also can detect enlarged perirectal lymph nodes, which may suggest nodal metastases; accuracy of detection of pathologically positive lymph nodes is 58% to 83%. Ultrasound may also prove useful for early detection of local recurrence after surgery.
Endoanal ultrasound is used to evaluate the layers of the anal canal. Internal anal sphincter, external anal sphincter, and puborectalis muscle can be differentiated. Endoanal ultrasound is particularly useful for detecting sphincter defects and for outlining complex anal fistulas.
Anorectal physiologic testing uses a variety of techniques to investigate the function of the pelvic floor. These techniques are useful in the evaluation of patients with incontinence, constipation, rectal prolapse, obstructed defecation, and other disorders of the pelvic floor.
Anorectal manometry is performed by placing a pressure-sensitive catheter in the lower rectum. The catheter is then withdrawn through the anal canal and pressures recorded. A balloon attached to the tip of the catheter also can be used to test anorectal sensation. The resting pressure in the anal canal reflects the function of the internal anal sphincter (normal, 40–80 mmHg), whereas the squeeze pressure, defined as the maximum voluntary contraction pressure minus the resting pressure, reflects function of the external anal sphincter (normal, 40–80 mmHg above resting pressure). The high-pressure zone estimates the length of the anal canal (normal, 2.0–4.0 cm). The rectoanal inhibitory reflex can be detected by inflating a balloon in the distal rectum; absence of this reflex is characteristic of Hirschsprung’s disease.
Neurophysiologic testing assesses function of the pudendal nerves and recruitment of puborectalis muscle fibers. Pudendal nerve terminal motor latency measures the speed of transmission of a nerve impulse through the distal pudendal nerve fibers (normal, 1.8–2.2 ms); prolonged latency suggests the presence of neuropathy. Electromyographic (EMG) recruitment assesses the contraction and relaxation of the puborectalis muscle during attempted defecation. Normally, recruitment increases when a patient is instructed to “squeeze” and decreases when a patient is instructed to “push.” Inappropriate recruitment is an indication of paradoxical contraction (nonrelaxation of the puborectalis). Needle EMG has been used to map both the pudendal nerves and the anatomy of the internal and external sphincters. However, this examination is painful and poorly tolerated by most patients. Needle EMG has largely been replaced by pudendal nerve motor latency testing to assess pudendal nerve function and endoanal ultrasound to map the sphincters.
Rectal evacuation studies include the balloon expulsion test and video defecography. Balloon expulsion assesses a patient’s ability to expel an intrarectal balloon. Video defecography provides a more detailed assessment of defecation. In this test, barium paste is placed in the rectum, and defecation is then recorded fluoroscopically. Defecography is used to differentiate nonrelaxation of the puborectalis, obstructed defecation, increased perineal descent, rectal prolapse and intussusception, rectocele, and enterocele. The addition of vaginal contrast and intraperitoneal contrast is useful in delineating complex disorders of the pelvic floor.
Fecal occult blood testing (FOBT) has been used as a screening test for colonic neoplasms in asymptomatic, average-risk individuals. The efficacy of this test is based on serial testing because the majority of colorectal malignancies will bleed intermittently. FOBT has been a nonspecific test for peroxidase contained in hemoglobin; consequently, occult bleeding from any gastrointestinal source will produce a positive result. Similarly, many foods (red meat, some fruits and vegetables, and vitamin C) will produce a false-positive result. Increased specificity is now possible by using immunochemical FOBT. These tests rely on monoclonal or polyclonal antibodies to react with the intact globin portion of human hemoglobin and are more specific for identifying occult bleeding from the colon or rectum. Any positive FOBT mandates further investigation, usually by colonoscopy.
Stool studies are often helpful in evaluating the etiology of diarrhea. Wet-mount examination reveals the presence of fecal leukocytes, which may suggest colonic inflammation or the presence of an invasive organism such as invasive E. coli or Shigella species. Stool cultures can detect pathogenic bacteria, ova, and/or parasites. C. difficile colitis is diagnosed by detecting bacterial toxin in the stool.10 Steatorrhea may be diagnosed by adding Sudan red stain to a stool sample.
Specific laboratory tests that should be performed will be dictated by the clinical scenario. Preoperative studies generally include a complete blood count and electrolyte panel. The addition of coagulation studies, liver function tests, and blood typing/cross-matching depends on the patient’s medical condition and the proposed surgical procedure.
Carcinoembryonic antigen (CEA) may be elevated in 60% to 90% of patients with colorectal cancer. Despite this, CEA is not an effective screening tool for this malignancy. Many practitioners follow serial CEA levels after curative-intent surgery in order to detect early recurrence of colorectal cancer. However, this tumor marker is nonspecific, and no survival benefit has yet been proven. It is also important to note that CEA may be mildly elevated in patients who smoke tobacco. Other biochemical markers (ornithine decarboxylase, urokinase) have been proposed, but none has yet proven sensitive or specific for detection, staging, or predicting prognosis of colorectal carcinoma.11
Although familial colorectal cancer syndromes, such as familial adenomatous polyposis (FAP) and hereditary nonpolyposis colon cancer (HNPCC) are rare, information about the specific genetic abnormalities underlying these disorders has led to significant interest in the role of genetic testing for colorectal cancer.12
Tests for mutations in the adenomatous polyposis coli (APC) gene responsible for FAP and in mismatch repair genes responsible for HNPCC are commercially available and extremely accurate in families with known mutations. However, in the absence of an identified mutation, a negative result is uninformative. For individuals from high-risk families without an identified mutation, increased surveillance is recommended.13 Although many of these mutations are also present in sporadic colorectal cancer, the accuracy of genetic testing in average-risk individuals is considerably lower, and these tests are not recommended for screening. Because of the potential psychosocial implications of genetic testing, it is strongly recommended that professional genetic counselors be involved in the care of any patient considering these tests.
Abdominal pain is a nonspecific symptom with myriad causes. Abdominal pain related to the colon and rectum can result from obstruction (either inflammatory or neoplastic), inflammation, perforation, or ischemia. Plain X-rays and judicious use of contrast studies and/or a CT scan can often confirm the diagnosis. Gentle retrograde contrast studies (Gastrografin enema) may be useful in delineating the degree of colonic obstruction. Sigmoidoscopy and/or colonoscopy performed by an experienced endoscopist can assist in the diagnosis of ischemic colitis, infectious colitis, and inflammatory bowel disease. However, if perforation or near complete obstruction is suspected, colonoscopy and/or sigmoidoscopy are generally contraindicated. Evaluation and treatment of abdominal pain from a colorectal source should follow the usual surgical principles of a thorough history and physical examination, appropriate diagnostic tests, resuscitation, and appropriately timed surgical intervention.
Pelvic pain can originate from the distal colon and rectum or from adjacent urogenital structures. Tenesmus may result from proctitis or from a rectal or retrorectal mass. Cyclical pain associated with menses, especially when accompanied by rectal bleeding, suggests a diagnosis of endometriosis. Pelvic inflammatory disease also can produce significant abdominal and pelvic pain. The extension of a peridiverticular abscess or periappendiceal abscess into the pelvis may also cause pain. CT scan and/or MRI may be useful in differentiating these diseases. Proctoscopy (if tolerated) also can be helpful. Occasionally, laparoscopy will yield a diagnosis.
Anorectal pain is most often secondary to an anal fissure, perirectal abscess and/or fistula, or a thrombosed hemorrhoid. Physical examination can usually differentiate these conditions. Other, less common causes of anorectal pain include anal canal neoplasms, perianal skin infection, and dermatologic conditions. Proctalgia fugax results from levator spasm and may present without any other anorectal findings. Physical exam is critical in evaluating patients with anorectal pain. If a patient is too tender to examine in the office, an examination under anesthesia is necessary. MRI or other imaging studies may be helpful in select cases where the etiology of pain is elusive.
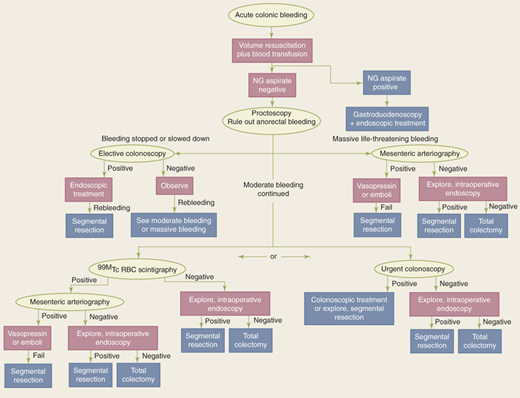
The first goal in evaluating and treating a patient with gastrointestinal hemorrhage is adequate resuscitation. The principles of ensuring a patent airway, supporting ventilation, and optimizing hemodynamic parameters apply, and coagulopathy and/or thrombocytopenia should be corrected. The second goal is to identify the source of hemorrhage. Because the most common source of gastrointestinal hemorrhage is esophageal, gastric, or duodenal, nasogastric aspiration should always be performed; return of bile suggests that the source of bleeding is distal to the ligament of Treitz. If aspiration reveals blood or nonbile secretions, or if symptoms suggest an upper intestinal source, esophagogastroduodenoscopy is performed. Anoscopy and/or limited proctoscopy can identify hemorrhoidal bleeding. A technetium-99 (99mTc)-tagged red blood cell (RBC) scan is extremely sensitive and is able to detect as little as 0.1 mL/h of bleeding; however, localization is imprecise. If the 99mTc-tagged RBC scan is positive, angiography can then be both diagnostic and potentially therapeutic. If the patient is hemodynamically stable, a rapid bowel preparation (over 4–6 hours) can be performed to allow colonoscopy. Colonoscopy may identify the cause of the bleeding, and cautery or injection of epinephrine into the bleeding site may be used to control hemorrhage. Colectomy may be required if bleeding persists despite these interventions. Intraoperative colonoscopy and/or enteroscopy may assist in localizing bleeding. If colectomy is required, a segmental resection is preferred if the bleeding source can be localized. “Blind” subtotal colectomy may very rarely be required in a patient who is hemodynamically unstable with ongoing colonic hemorrhage of an unknown source. In this setting, just prior to proceeding with a “blind” subtotal colectomy, it is crucial to irrigate the rectosigmoid and re-examine the mucosa of the anal canal and rectum by anoscopy and proctoscopy to ensure the source of ongoing bleeding is not distal to the planned resection margin (Fig. 29-7).
Figure 29-7.
Algorithm for treatment of colorectal hemorrhage. NG = nasogastric; 99mTc = technetium-99; RBC = red blood cell. (Reproduced with permission of Taylor & Francis, LLC from Gordon PH, Nivatvongs S, eds. Principles and Practice of Surgery for the Colon, Rectum, and Anus. 2nd ed. New York: Marcel Dekker, Inc.; 1999:1279. Permission conveyed through Copyright Clearance Center, Inc.)
Occult blood loss from the gastrointestinal tract may manifest as iron-deficiency anemia or may be detected with FOBT. Because colon neoplasms bleed intermittently and rarely present with rapid hemorrhage, the presence of occult fecal blood should always prompt a colonoscopy. Unexplained iron-deficiency anemia is also an indication for colonoscopy.
Hematochezia is commonly caused by hemorrhoids or a fissure. Sharp, knife-like pain and bright red rectal bleeding with bowel movements suggest the diagnosis of fissure. Painless, bright red rectal bleeding with bowel movements is often secondary to a friable internal hemorrhoid that is easily detected by anoscopy. In the absence of a painful, obvious fissure, any patient with rectal bleeding should undergo a careful digital rectal examination, anoscopy, and proctosigmoidoscopy. Failure to diagnose a source in the distal anorectum should prompt colonoscopy.
Constipation is an extremely common complaint, affecting more than 4 million people in the United States. Despite the prevalence of this problem, there is lack of agreement about an appropriate definition of constipation. Patients may describe infrequent bowel movements, hard stools, or excessive straining. A careful history of these symptoms often clarifies the nature of the problem.
Constipation has many causes. Underlying metabolic, pharmacologic, endocrine, psychological, and neurologic causes often contribute to the problem. A stricture or mass lesion should be excluded by colonoscopy, barium enema, or CT colonography. After these causes have been excluded, evaluation focuses on differentiating slow-transit constipation from outlet obstruction. Transit studies, in which radiopaque markers are swallowed and then followed radiographically, are useful for diagnosing slow-transit constipation. Anorectal manometry and EMG can detect nonrelaxation of the puborectalis, which contributes to outlet obstruction. The absence of an anorectal inhibitory reflex suggests Hirschsprung’s disease and may prompt a rectal mucosal biopsy. Defecography can identify rectal prolapse, intussusception, rectocele, or enterocele.
Medical management is the mainstay of therapy for constipation and includes fiber, increased fluid intake, and laxatives. Outlet obstruction from nonrelaxation of the puborectalis often responds to biofeedback.14 Surgery to correct rectocele and rectal prolapse has a variable effect on symptoms of constipation but can be successful in selected patients. Subtotal colectomy is considered only for patients with severe slow-transit constipation (colonic inertia) refractory to maximal medical interventions. While this operation almost always increases bowel movement frequency, complaints of diarrhea, incontinence, and abdominal pain are not infrequent, and patients should be carefully selected and counseled.15
Diarrhea is also a common complaint and is usually a self-limited symptom of infectious gastroenteritis. If diarrhea is chronic or is accompanied by bleeding or abdominal pain, further investigation is warranted. Bloody diarrhea and pain are characteristic of colitis; etiology can be an infection (invasive E. coli, Shigella, Salmonella, Campylobacter, Entamoeba histolytica, or C. difficile), inflammatory bowel disease (ulcerative colitis or Crohn’s colitis), or ischemia. Stool wet-mount and culture can often diagnose infection. Sigmoidoscopy or colonoscopy can be helpful in diagnosing inflammatory bowel disease or ischemia. However, if the patient has abdominal tenderness, particularly with peritoneal signs, or any other evidence of perforation, endoscopy is contraindicated.
Chronic diarrhea may present a more difficult diagnostic dilemma. Chronic ulcerative colitis, Crohn’s colitis, infection, malabsorption, and short gut syndrome can cause chronic diarrhea. Rarely, carcinoid syndrome and islet cell tumors (vasoactive intestinal peptide–secreting tumor [VIPoma], somatostatinoma, gastrinoma) present with this symptom. Large villous lesions may cause secretory diarrhea. Collagenous colitis can cause diarrhea without any obvious mucosal abnormality. Along with stool cultures, tests for malabsorption, and metabolic investigations, colonoscopy can be invaluable in differentiating these causes. Biopsies should be taken even if the colonic mucosa appears grossly normal.
Irritable bowel syndrome is a particularly troubling constellation of symptoms consisting of crampy abdominal pain, bloating, constipation, and urgent diarrhea. Workup reveals no underlying anatomic or physiologic abnormality. Once other disorders have been excluded, dietary restrictions and avoidance of caffeine, alcohol, and tobacco may help to alleviate symptoms. Antispasmodics and bulking agents may be helpful.
The incidence of fecal incontinence has been estimated to occur in 10 to 13 individuals per 1000 people older than age 65 years. Incontinence ranges in severity from occasional leakage of gas and liquid stool to daily loss of solid stool. The underlying cause of incontinence is often multifactorial, and diarrhea is often contributory. In general, causes of incontinence can be classified as neurogenic or anatomic. Neurogenic causes include diseases of the central nervous system and spinal cord along with pudendal nerve injury. Anatomic causes include congenital abnormalities, procidentia (rectal prolapse), overflow incontinence secondary to impaction or an obstructing neoplasm, and trauma. The most common traumatic cause of incontinence is injury to the anal sphincter during vaginal delivery. Other causes include anorectal surgery, impalement, and pelvic fracture.
After a thorough medical evaluation to detect underlying conditions that might contribute to incontinence, evaluation focuses on assessment of the anal sphincter and pudendal nerves. Pudendal nerve terminal motor latency testing may detect neuropathy. Anal manometry can detect low resting and squeeze pressures. Physical examination and defecography can detect rectal prolapse. Endoanal ultrasound is invaluable in diagnosing sphincter defects (Fig. 29-8).
Figure 29-8.
A. Endoanal ultrasonography showing the normal layers of the anal canal. B. Endoanal ultrasonography with anterior sphincter defect from birthing injury. EAS = external anal sphincter; IAS = internal anal sphincter. (Both images used with permission of Charles O. Finne III, MD, Minneapolis, MN.)
Therapy depends on the underlying abnormality. Diarrhea should be treated medically (fiber, antidiarrheal agents). Even in the absence of frank diarrhea, the addition of dietary fiber may improve continence. Some patients may respond to biofeedback. Many patients with a sphincter defect are candidates for an overlapping sphincteroplasty. Innovative technologies such as sacral nerve stimulation and the artificial bowel sphincter are proving useful in patients who fail other interventions.16 The delivery of radiofrequency energy to the anal canal (the Secca procedure) appears to be safe and effective in early studies.17 Injectable bulking agents are also gaining popularity, but long-term efficacy has not yet been proven.18 Finally, a stoma can provide relief for severely incontinent patients who have failed or are not candidates for other interventions.19
GENERAL SURGICAL CONSIDERATIONS
Colorectal resections are performed for a wide variety of conditions, including neoplasms (benign and malignant), inflammatory bowel diseases, and other benign conditions. Although the indication and urgency for surgery will alter some of the technical details, the operative principles of colorectal resections, anastomoses, and use of ostomies are well established.
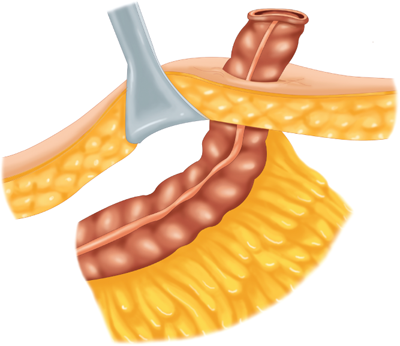
The mesenteric clearance technique dictates the extent of colonic resection and is determined by the nature of the primary pathology (malignant or benign), the intent of the resection (curative or palliative), the precise location(s) of the primary pathology, and the condition of the mesentery (thin and soft or thick and indurated). In general, a proximal mesenteric ligation will eliminate the blood supply to a greater length of colon and require a more extensive “colectomy.” Curative resection of a colorectal cancer is usually best accomplished by performing a proximal mesenteric vessel ligation and radical mesenteric clearance of the lymphatic drainage basin of the tumor site (Fig. 29-9). Resection of a benign process does not require wide mesenteric clearance.
Emergency resection may be required because of obstruction, perforation, or hemorrhage. In this setting, the bowel is almost always unprepared and the patient may be unstable. The surgical principles described earlier apply, and an attempt should be made to resect the involved segment along with its lymphovascular supply. If the resection involves the right colon or proximal transverse colon (right or extended right colectomy), a primary ileocolonic anastomosis can usually be performed safely as long as the remaining bowel appears healthy and the patient is stable. For left-sided tumors, the traditional approach has involved resection of the involved bowel and end colostomy, with or without a mucus fistula. However, there is an increasing body of data to suggest that a primary anastomosis without a bowel preparation or with an on-table lavage, with or without a diverting ileostomy, may be equally safe in this setting. If the proximal colon appears unhealthy (vascular compromise, serosal tears, perforation), a subtotal colectomy can be performed with a small bowel to rectosigmoid anastomosis. Resection and diversion (ileostomy or colostomy) remain safe and appropriate if the bowel appears compromised or if the patient is unstable, malnourished, or immunosuppressed.
With advances in minimally invasive technology, many procedures that previously have required laparotomy can now be performed laparoscopically,20,21 with hand-assisted laparoscopy (HAL), or robotically. Potential advantages of minimally invasive surgery include improved cosmetic result, decreased postoperative pain, and earlier return of bowel function. Moreover, some experimental data suggest that minimally invasive operations have less immunosuppressive impact on the patient and thus might improve postoperative outcome and even long-term survival. To date, most studies have demonstrated equivalence between laparoscopic, HAL, and open resection in terms of extent of resection. Return of bowel function and length of hospital stay are highly variable. Long-term outcome has yet to be determined; however, short-term quality of life appears to be improved by laparoscopy.22,23 The most recent advances in minimally invasive surgery involve use of robotics and telemanipulation in which the surgeon operates from a console remote from the patient. These procedures have been rapidly gaining in popularity, especially for pelvic and rectal resections. Early studies suggest equivalence between robotic resections and laparoscopic/HAL resections.24
In addition, some proponents have suggested that robotic procedures may be easier to learn (a shorter “learning curve”) and that robotic surgery may be ergonomically better for the operating surgeon. Nevertheless, long-term superiority, or even equivalence, has yet to be demonstrated, and these advanced technologies are likely to be associated with significant cost.
A variety of terms are used to describe different types of colectomy (Fig. 29-10).
Figure 29-10.
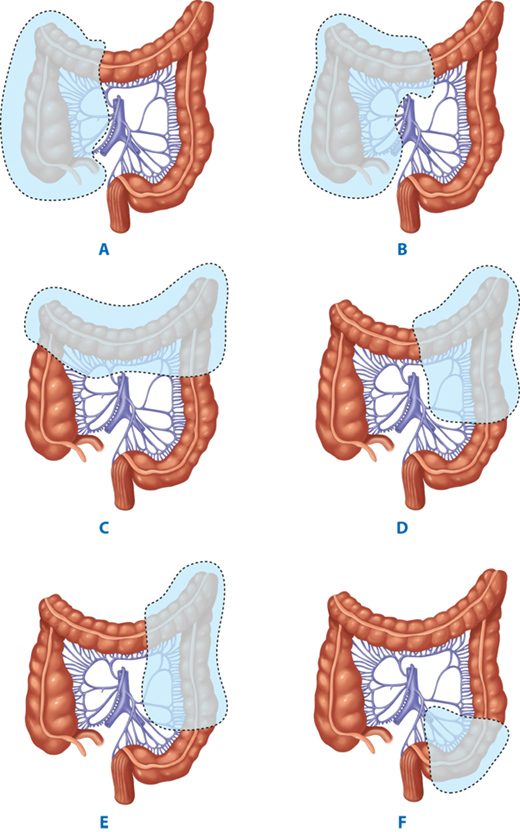
Terminology of types of colorectal resections: A→C Ileocecectomy; + A + B→D Ascending colectomy; + A + B→F Right hemicolectomy; + A + B→G Extended right hemicolectomy; + E + F→G + H Transverse colectomy; G→I Left hemicolectomy; F→I Extended left hemicolectomy; J + K Sigmoid colectomy; + A + B→J Subtotal colectomy; + A + B→K Total colectomy; + A + B→L Total proctocolectomy. (Reproduced with permission from Fielding LP, Goldberg SM, eds. Rob & Smith’s Operative: Surgery of the Colon, Rectum, and Anus. London: Elsevier Science Ltd; 1993:349.)
An ileocolic resection describes a limited resection of the terminal ileum, cecum, and appendix. It is used to remove disease involving these segments of the intestine (e.g., ileocecal Crohn’s disease) and benign lesions or incurable cancers arising in the terminal ileum, cecum, and, occasionally, the appendix. If curable malignancy is suspected, more radical resections, such as a right hemicolectomy, are generally indicated. The ileocolic vessels are ligated and divided. A variable length of small intestine may be resected depending on the disease process. A primary anastomosis is created between the distal small bowel and the ascending colon. It is technically difficult to perform an anastomosis at or just proximal to the ileocecal valve; therefore, if the most distal ileum needs to be resected, the cecum is generally also removed.
A right colectomy is used to remove lesions or disease in the right colon and is oncologically the most appropriate operation for curative intent resection of proximal colon carcinoma. The ileocolic vessels, right colic vessels, and right branches of the middle colic vessels are ligated and divided. Approximately 10 cm of terminal ileum are usually included in the resection. A primary ileal-transverse colon anastomosis is almost always possible.
An extended right colectomy may be used for curative intent resection of lesions located at the hepatic flexure or proximal transverse colon. A standard right colectomy is extended to include ligation of the middle colic vessels at their base. The right colon and proximal transverse colon are resected, and a primary anastomosis is created between the distal ileum and distal transverse colon. Such an anastomosis relies on the marginal artery of Drummond. If the blood supply to the distal transverse colon is questionable, the resection is extended distally beyond the splenic flexure to well-perfused descending colon where the ileocolic anastomosis can be performed safely.
Lesions in the mid and distal transverse colon may be resected by ligating the middle colic vessels and resecting the transverse colon, followed by a colocolonic anastomosis. However, an extended right colectomy with an anastomosis between the terminal ileum and descending colon may be a safer anastomosis with an equivalent functional result.
For lesions or disease states confined to the distal transverse colon, splenic flexure, or descending colon, a left colectomy is performed. The left branches of the middle colic vessels, the left colic vessels, and the first branches of the sigmoid vessels are ligated. A colocolonic anastomosis can usually be performed.
An extended left colectomy is an option for removing lesions in the distal transverse colon. In this operation, the left colectomy is extended proximally to include the right branches of the middle colic vessels.
Lesions in the sigmoid colon require ligation and division of the sigmoid branches of the inferior mesenteric artery. In general, the entire sigmoid colon should be resected to the level of the peritoneal reflection and an anastomosis created between the descending colon and upper rectum. Full mobilization of the splenic flexure is often required to create a tension-free anastomosis.
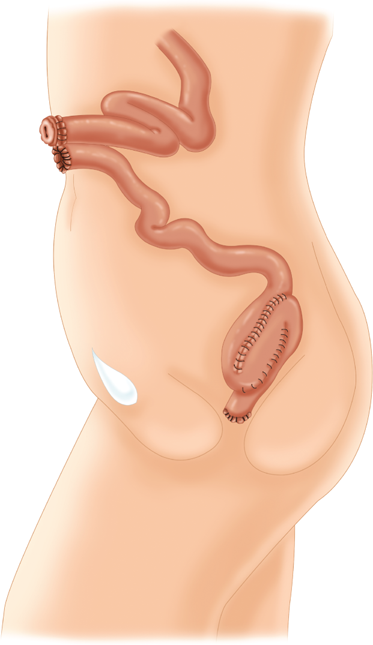
Total or subtotal colectomy is occasionally required for patients with fulminant colitis, attenuated FAP, or synchronous colon carcinomas. In this procedure, the ileocolic vessels, right colic vessels, middle colic vessels, and left colic vessels are ligated and divided. The superior rectal vessels are preserved. If it is desired to preserve the sigmoid, the distal sigmoid vessels are left intact, and an anastomosis is created between the ileum and distal sigmoid colon (subtotal colectomy with ileosigmoid anastomosis). If the sigmoid is to be resected, the sigmoidal vessels are ligated and divided, and the ileum is anastomosed to the upper rectum (total abdominal colectomy with ileorectal anastomosis). If an anastomosis is contraindicated, an end ileostomy is created, and the remaining sigmoid or rectum is managed either as a mucus fistula or a Hartmann’s pouch.
In this procedure, the entire colon, rectum, and anus are removed and the ileum is brought to the skin as a Brooke ileostomy.
The entire colon and rectum are resected, but the anal sphincter muscles and a variable portion of the distal anal canal are preserved. Bowel continuity is restored by anastomosis of an ileal reservoir to the anal canal. The original technique included a transanal mucosectomy and hand-sewn ileoanal anastomosis. Proponents of this technique argue that mucosectomy guarantees removal of all of the diseased mucosa, including the anal transition zone, and therefore decreases the risk of ongoing disease, dysplasia, and carcinoma.25 Opponents cite the increased risk of incontinence after mucosectomy and argue that even meticulous technique invariably leaves behind mucosal “islands” that are subsequently hidden under the anastomosis. Moreover, the “double-staple” technique using the circular stapling devices is considerably simpler than mucosectomy and a hand-sewn anastomosis and may be associated with a better functional outcome (Fig. 29-11).26,27,28,29 Regardless of the anastomotic technique, many surgeons recommend that patients undergo annual surveillance of the anastomosis and/or anal transition zone by digital rectal exam and anoscopy or proctoscopy.
Figure 29-11.
After a total colectomy and resection of the rectum (A), the anal canal with a short cuff of transitional mucosa and sphincter muscles is preserved (B). An ileal J-pouch has been constructed and is anastomosed to the anal canal using a double-staple technique (C). (Reproduced with permission from Bell RH, Rikkers LF, Mulholland M, eds. Digestive Tract Surgery: A Text and Atlas. Philadelphia: Lippincott Williams & Wilkins; 1996:1527.)
The neorectum is made by anastomosis of the terminal ileum aligned in a “J,” “S,” or “W” configuration. Because functional outcomes are similar and because the J-pouch is the simplest to construct, it has become the most used configuration. With increasing experience in laparoscopic and robotic colectomy, some centers have begun performing total proctocolectomy with ileal pouch–anal reconstruction using minimally invasive surgical techniques.20 Most surgeons perform a proximal ileostomy to divert succus from the newly created pouch in an attempt to minimize the consequences of leak and sepsis, especially in patients who are malnourished or immunosuppressed (Fig. 29-12). The ileostomy is then closed 6 to 12 weeks later, after a contrast study confirms the integrity of the pouch. In low-risk patients, however, there are reports of successful creation of an ileoanal pouch without a diverting stoma.
Anterior resection is the general term used to describe resection of the rectum from an abdominal approach to the pelvis with no need for a perineal, sacral, or other incision. Three types of anterior resection have been described.
A high anterior resection is the term used to describe resection of the distal sigmoid colon and upper rectum and is the appropriate operation for benign lesions and disease at the rectosigmoid junction such as diverticulitis. The upper rectum is mobilized, but the pelvic peritoneum is not divided and the rectum is not mobilized fully from the concavity of the sacrum. The inferior mesenteric artery is ligated at its base, and the inferior mesenteric vein, which follows a different course than the artery, is ligated separately. A primary anastomosis (usually end-to-end) between the colon and rectal stump with a short cuff of peritoneum surrounding its anterior two thirds generally can be performed.
A low anterior resection is used to remove lesions in the upper and mid rectum. The rectosigmoid is mobilized, the pelvic peritoneum is opened, and the inferior mesenteric artery is ligated and divided either at its origin from the aorta or just distal to the takeoff of the left colic artery. The rectum is mobilized from the sacrum by sharp dissection under direct view within the endopelvic fascial plane. The dissection may be performed distally to the anorectal ring, extending posteriorly through the rectosacral fascia to the coccyx and anteriorly through Denonvilliers’ fascia to the vagina in women or the seminal vesicles and prostate in men. The rectum and accompanying mesorectum are divided at the appropriate level, depending on the nature of the lesion. A low rectal anastomosis usually requires mobilization of the splenic flexure and ligation and division of the inferior mesenteric vein just inferior to the pancreas. Circular stapling devices have greatly facilitated the conduct and improved the safety of the colon to extraperitoneal rectal anastomosis.
An extended low anterior resection is necessary to remove lesions located in the distal rectum, but several centimeters above the sphincter. The rectum is fully mobilized to the level of the levator ani muscle just as for a low anterior resection, but the anterior dissection is extended along the rectovaginal septum in women and distal to the seminal vesicles and prostate in men. After resection at this level, a coloanal anastomosis can be created using one of a variety of techniques. An end-to-end stapled or hand-sewn anastomosis has traditionally been the procedure of choice. However, the functional consequences of a “straight” anastomosis have led to consideration for creation of a colon J-pouch or transverse coloplasty to increase the capacity of the neorectal reservoir.30 Because the risk of an anastomotic leak and subsequent sepsis is higher when an anastomosis is created in the distal rectum or anal canal, creation of a temporary ileostomy should be considered in this setting.
Although an anastomosis may be technically feasible very low in the rectum or anal canal, it is important to note that postoperative function may be poor. Because the descending colon lacks the distensibility of the rectum, the reservoir function may be compromised. Pelvic radiation, prior anorectal surgery, and obstetrical trauma may cause unsuspected sphincter damage. Finally, a very low anastomosis may involve and compromise the upper sphincter. Creation of a colon J-pouch or transverse coloplasty may improve function, but few long-term studies have addressed this issue.30,31
A history of sphincter damage or any degree of incontinence is a relative contraindication for a coloanal anastomosis. In such patients, an end colostomy may be a more satisfactory option.
Hartmann’s procedure refers to a colon or rectal resection without an anastomosis in which a colostomy or ileostomy is created and the distal colon or rectum is left as a blind pouch. The term is typically used when the left or sigmoid colon is resected and the closed off rectum is left in the pelvis. If the distal colon is long enough to reach the abdominal wall, a mucus fistula can be created by opening the defunctioned bowel and suturing the open lumen to the skin.
An abdominoperineal resection (APR) involves removal of the entire rectum, anal canal, and anus with construction of a permanent colostomy from the descending or sigmoid colon. The abdominal-pelvic portion of this operation proceeds in the same fashion as described for an extended low anterior resection. The perineal dissection can be performed with the patient in lithotomy position (often by a second surgeon) or in the prone position after closure of the abdomen and creation of the colostomy. For cancer, the perineal dissection is designed to excise the anal canal with a wide circumferential margin including a cylindrical cuff of the levator muscle. Primary wound closure is usually successful, but a large perineal defect, especially if preoperative radiation has been used, may require a vascularized flap closure in some patients. For benign disease, proctectomy may be performed using an intersphincteric dissection between the internal and external sphincters. This approach minimizes the perineal wound, making it easier to close because the levator muscle remains intact.
Anastomoses may be created between two segments of bowel in a multitude of ways. The geometry of the anastomosis may be end-to-end, end-to-side, side-to-end, or side-to-side. The anastomotic technique may be hand-sewn or stapled (Fig. 29-13). The submucosal layer of the intestine provides the strength of the bowel wall and must be incorporated in the anastomosis to assure healing. The choice of anastomosis depends on the operative anatomy and surgeon preference. Although many surgeons advocate one method over another, none has been proven to be superior. Accurate approximation of two well-vascularized, healthy limbs of bowel without tension in a normotensive, well-nourished patient almost always results in a good outcome. Anastomoses at highest risk of leak or stricture are those that are in the distal rectal or anal canal, involve irradiated or diseased intestine including perforation with peritoneal soilage, or are performed in malnourished, immunosuppressed, or ill patients.
Figure 29-13.
A. Sutured end-to-end colocolic anastomosis. B. Sutured end-to-side ileocolic anastomosis. C. Stapled side-to-side, functional end-to-end ileocolic anastomosis. (Reproduced with permission from Bell RH, Rikkers LF, Mulholland M, eds. Digestive Tract Surgery: A Text and Atlas. Philadelphia: Lippincott Williams & Wilkins; 1996:1473, 1475, and 1479.)
An end-to-end anastomosis can be performed when two segments of bowel are roughly the same caliber. This technique is most often employed in rectal resections, but may be used for colocolostomy or small bowel anastomoses.
An end-to-side configuration is useful when one limb of bowel is larger than the other. This most commonly occurs in the setting of chronic obstruction.
A side-to-end anastomosis is used when the proximal bowel is of smaller caliber than the distal bowel. Ileorectal anastomoses commonly make use of this configuration. A side-to-end anastomosis may have a less tenuous blood supply than an end-to-end anastomosis.
A side-to-side anastomosis allows a large, well-vascularized connection to be created on the antimesenteric side of two segments of intestine. This technique is commonly used in ileocolic and small bowel anastomoses.
Any of the configurations described earlier may be created using a hand-sutured or stapled technique. Hand-sutured anastomoses may be single layer, using either running or interrupted stitches, or double layer. A double-layer anastomosis usually consists of a continuous inner layer and an interrupted outer layer. Suture material may be either permanent or absorbable. After distal rectal or anal canal resection, a transanal, hand-sewn coloanal anastomosis may be necessary to restore bowel continuity. This can be done in conjunction with an anal canal mucosectomy to allow the anastomosis to be created at the dentate line.
Linear cutting/stapling devices are used to divide the bowel and to create side-to-side anastomoses. The anastomosis may be reinforced with interrupted sutures if desired. Circular cutting/stapling devices can create end-to-end, end-to-side, or side-to-end anastomoses. These instruments are particularly useful for creating low rectal or anal canal anastomoses where the anatomy of the pelvis makes a hand-sewn anastomosis technically difficult or impossible.
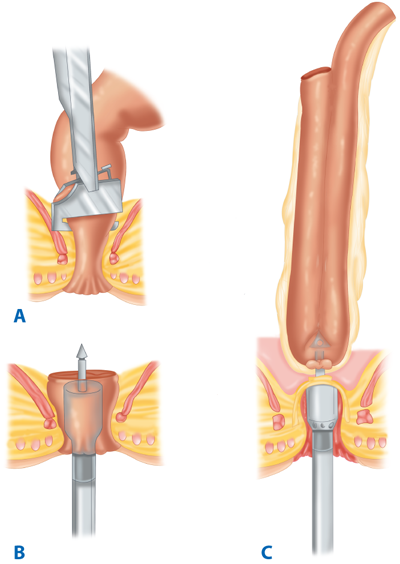
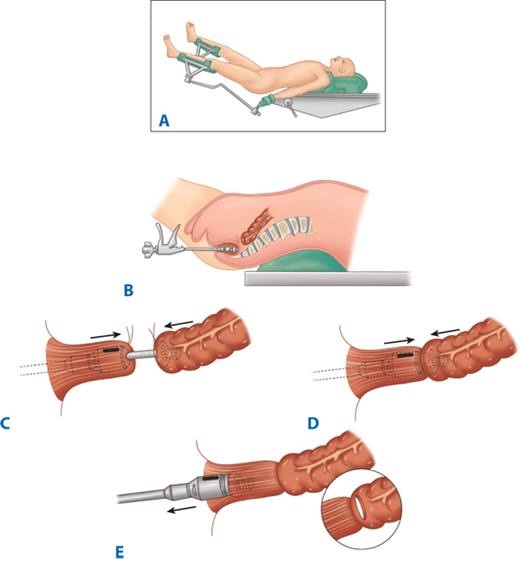
Following resection of the colorectum, a stapled end-to-end colorectal, coloanal canal, or ileal pouch–anal canal anastomosis may be created by one of two techniques. With the open purse-string technique, the distal rectal stump purse-string is placed by hand, and the assembled circular stapler is inserted into the anus and guided up to the rectal purse-string. The stapler is opened, and the distal purse-string is tied. A purse-string is placed in the distal end of the proximal colon; the proximal colon is placed over the anvil and the purse-string tightened. The stapler is closed and fired (Fig. 29-14). With the alternative double-staple technique, the distal rectum or anal canal is closed with a transverse staple line. The circular stapler is inserted through the anus without its anvil until the cartridge effaces the transverse staple line. The stapler is opened, causing the trocar to perforate through the rectal stump adjacent to the transverse staple line. The anastomosis in then completed as described earlier (see Fig. 29-11). After firing and removing the stapler, the resulting anastomotic rings should be inspected to ensure that they are intact. A gap in an anastomotic ring suggests that the circular staple line is incomplete and the anastomosis should be reinforced with suture circumferentially, if technically feasible. A temporary proximal ileostomy may be indicated as well. Most surgeons will also leak test an anastomosis by instilling water or saline into the pelvis and insufflating the rectum with air via a proctoscope or alternatively instilling methylene blue or betadine into the rectum to look for extravasation.
Figure 29-14.
Technique of end-to-end colorectal anastomosis using a circular stapler. A. The patient is in modified lithotomy position. B. After resection of the rectosigmoid and placement of purse-string sutures proximally and distally, the stapler is inserted into the anal canal and opened. C. Rectal purse-string suture is tied to secure the rectal stump to the rod of the stapler, and the colonic purse-string is tied to secure the colon to the anvil of the stapler. D. The stapler is closed and fired. E. The stapler is removed, leaving a circular stapled end-to-end anastomosis.
Depending on the clinical situation, a stoma may be temporary or permanent. It may be end-on or a loop. However, regardless of the indication for a stoma, placement and construction are crucial for function.
The preoperative preparation of a patient who is expected to require a stoma should include a consultation with an enterostomal therapy (ET) nurse. ET nurses are specially trained and credentialed by the Wound, Ostomy and Continence Nurses Society. Preoperative planning includes counseling, education, and stoma siting. Postoperatively, the ET nurse assists with local skin care and pouching. Other considerations in stoma planning include evaluation of other medical conditions that may impact on a patient’s ability to manage a stoma (e.g., eyesight, manual dexterity).
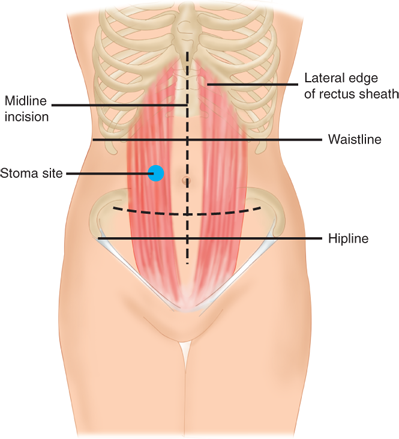
Preoperative stoma siting is crucial for a patient’s postoperative function and quality of life. A poorly placed stoma can result in leakage and skin breakdown. Ideally, a stoma should be placed in a location that the patient can easily see and manipulate, within the rectus muscle, and below the belt line (see Fig. 29-15). Because the abdominal landmarks in a supine, anesthetized patient may be dramatically different from those in an awake, standing, or sitting patient, the stoma site should always be marked with a tattoo, skin scratch, or permanent marker preoperatively, if possible. In an emergency operation where the stoma site has not been marked, an attempt should be made to place a stoma within the rectus muscle and away from both the costal margin and iliac crest. In emergencies, placement high on the abdominal wall is preferred to a low-lying site.
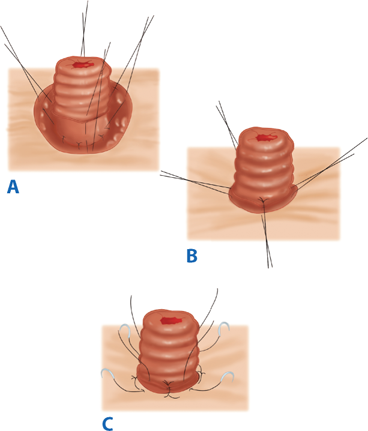
For all stomas, a circular skin incision is created and the subcutaneous tissue dissected to the level of the anterior rectus sheath. The anterior rectus sheath is incised in a cruciate fashion, the muscle fibers separated bluntly, and the posterior sheath identified and incised. Care should be taken to avoid injuring and causing bleeding from the inferior epigastric artery and vein. The size of the defect depends on the size of the bowel used to create the stoma, but should be as small as possible without compromising the intestinal blood supply (usually the width of two to three fingers). The bowel is then brought through the defect and secured with sutures. The abdominal incision is usually closed and dressed prior to maturing the stoma to avoid contaminating the wound. In order to make appliance use easier, a protruding nipple is fashioned by everting the bowel. Three or four interrupted absorbable sutures are placed through the edge of the bowel, then through the serosa, approximately 2 cm proximal to the edge, and then through the dermis (Brooke technique). After the stoma is everted, the mucocutaneous junction is sutured circumferentially with interrupted absorbable suture (Fig. 29-16).
Figure 29-16.
Brooke ileostomy. A. Four sutures incorporating the cut end of the ileum, the seromuscular layer at the level of the anterior rectus fascia, and the subcuticular edge of the skin are placed at 90° to each other. B. The sutures are tied to produce stomal eversion, and (C) simple sutures from the cut edge of the bowel to the subcuticular tissue complete the maturation of the ileostomy. (Reproduced with permission from Bell RH, Rikkers LF, Mulholland M, eds. Digestive Tract Surgery: A Text and Atlas. Philadelphia: Lippincott Williams & Wilkins; 1996:1278.)
A temporary ileostomy is often used to “protect” an anastomosis that is at risk for leakage (low in the rectum, in an irradiated field, in an immunocompromised or malnourished patient, and during some emergency operations). In this setting, the stoma is often constructed as a loop ileostomy (see Fig. 29-12). A segment of distal ileum is brought through the defect in the abdominal wall as a loop. An enterotomy is created and the stoma matured as described earlier. The loop may be secured with or without an underlying rod. A divided loop may also be created by firing a linear cutting/stapler across the distal limb of the loop flush with the skin followed by maturation of the proximal limb of the loop. This technique prevents incomplete diversion that occasionally occurs with a loop ileostomy.
The advantage of a loop or divided loop ileostomy is that subsequent closure can often be accomplished without a formal laparotomy. An elliptical incision is created around the stoma and the bowel gently dissected free of the subcutaneous tissues and fascia. A hand-sewn or stapled anastomosis can then be created and the intestine returned to the peritoneal cavity. This avoids a long laparotomy incision and generally is well tolerated. The timing of ileostomy closure should take into account anastomotic healing as well as the patient’s overall condition. A flexible endoscopy exam and a contrast enema (Gastrografin) are recommended prior to closure to ensure that the anastomosis has not leaked and is patent. A patient’s nutritional status should be optimized. In cancer patients receiving adjuvant chemotherapy, ileostomy closure should be delayed until the chemotherapy is completed.
A permanent ileostomy is sometimes required after total proctocolectomy or in patients with obstruction. An end ileostomy is the preferred configuration for a permanent ileostomy because a symmetric protruding nipple can be fashioned more easily than with a loop ileostomy (see Fig. 29-16). The end of the small intestine is brought through the abdominal wall defect and matured. Stitches are often used to secure the bowel to the posterior fascia.
Stoma necrosis may occur in the early postoperative period and is usually caused by skeletonizing the distal small bowel and/or creating an overly tight fascial defect. Limited mucosal necrosis above the fascia may be treated expectantly, but necrosis below the level of the fascia requires surgical revision. Stoma retraction may occur early or late and may be exacerbated by obesity. Local revision may be necessary. The creation of an ileostomy bypasses the fluid-absorbing capability of the colon, and dehydration with fluid and electrolyte abnormalities is not uncommon. Ideally, ileostomy output should be maintained at less than 1500 mL/d to avoid this problem. Bulk agents and opioids (Lomotil, Imodium, tincture of opium) are useful. The somatostatin analogue, octreotide, has been used with varying success in this setting. Skin irritation can also occur, especially if the stoma appliance fits poorly. Skin-protecting agents and custom pouches can help to solve this problem. Obstruction may occur intra-abdominally or at the site where the stoma exits the fascia. Parastomal hernia is less common after an ileostomy than after a colostomy but can cause poor appliance fitting, pain, obstruction, or strangulation. In general, symptomatic parastomal hernias should be repaired. A variety of techniques to repair these hernias have been described, including local repair (either with or without mesh), laparoscopic repair, and stoma resiting. Prolapse is a rare, late complication and is often associated with a parastomal hernia.
Most colostomies are created as end colostomies rather than loop colostomies (Fig. 29-17). The bulkiness of the colon makes a loop colostomy awkward for use of an appliance, and prolapse is more likely with this configuration. Most colostomies are created on the left side of the colon. An abdominal wall defect is created and the end of the colon mobilized through it. Because a protruding stoma is considerably easier to pouch, colostomies should also be matured in a Brooke fashion. The distal bowel may be brought through the abdominal wall as a mucus fistula or left intra-abdominally as a Hartmann’s pouch. Tacking the distal end of the colon to the abdominal wall or tagging it with permanent suture can make identification of the stump easier if the colostomy is closed at a later date. Closure of an end colostomy has traditionally required a laparotomy, but increasingly minimally invasive techniques have been adopted. The stoma is dissected free of the abdominal wall and the distal bowel identified. An end-to-end anastomosis is then created.
Colostomy necrosis may occur in the early postoperative period and results from an impaired vascular supply (skeletonization of the distal colon or a tight fascial defect). Like ileostomy necrosis, limited suprafascial necrosis may be followed expectantly, but necrosis below the fascia requires surgery. Retraction may also occur but is less problematic with a colostomy than with an ileostomy because the stool is less irritating to the skin than succus entericus. Obstruction is unusual, but may also occur. Parastomal hernia is the most common late complication of a colostomy and requires repair if it is symptomatic. Prolapse occurs rarely, but is more common with a loop colostomy. Interestingly, it is almost always the efferent limb of the loop that prolapses. Dehydration is rare after colostomy, and skin irritation is less common than with ileostomy.
Function following segmental colonic resection and primary anastomosis is generally excellent. A small percentage of patients following subtotal or total colectomy and ileosigmoid or ileorectal anastomosis may experience diarrhea and bowel frequency. This is especially true if the patient is elderly, if significant length of small bowel has been resected, and if residual proctocolitis is poorly controlled. In general, the more distal the anastomosis, the greater is the risk of troublesome diarrhea and frequency. However, some patients develop significant diarrhea after right colectomy due to malabsorption of bile acids; in these cases, bile acid binding resins (e.g., cholestyramine) sometimes can be helpful.
Function following anterior resection is highly dependent on the level of anastomosis, the use of pre- or postoperative pelvic radiation, and underlying sphincter function. Following low anterior or extended low anterior resection, some surgeons prefer to construct a short (5-cm) colon J-pouch to anastomose to the distal rectum or anal canal or, alternatively, a transverse coloplasty to increase the capacity of the neorectum. The reservoirs are thought to lessen urgency, frequency, and incontinence, but some patients have difficulty initiating defecation, and long-term superiority over a “straight” anastomosis has yet to be proven. In addition, these reservoirs can be technically difficult, especially in an obese male with a narrow pelvis.
The physical and psychological problems associated with a permanent Brooke ileostomy led to development of the continent Kock pouch ileostomy. Unfortunately, complications, especially complications related to valve slippage, are common. Despite variations of technique designed to improve the function of the continent ileostomy, most surgeons have abandoned this operation and instead perform restorative proctocolectomy with ileal pouch–anal anastomosis.
Although ileal pouch–anal reconstruction is anatomically appealing, functional outcome is far from perfect.6,25 Patients should be counseled to expect 8 to 10 bowel movements per day. Up to 50% have some degree of nocturnal incontinence. Pouchitis occurs in nearly 50% of patients who undergo the operation for chronic ulcerative colitis, and small bowel obstruction is not uncommon. Other less common complications include difficulties with pouch evacuation, pouch-anal and/or pouch-vaginal fistula, and anal stricture. Pouch failure rate averages 5% to 10%. Patients who are subsequently diagnosed with Crohn’s disease have a considerably higher pouch failure rate (approximately 50%), whereas patients with indeterminate colitis have a lower pouch failure rate (15%–20%). Despite these drawbacks, the vast majority of patients are satisfied and prefer ileal pouch–anal reconstruction to permanent ileostomy.
Pouchitis is an inflammatory condition that affects both ileoanal pouches and continent ileostomy reservoirs. The incidence of pouchitis ranges from 30% to 55%. Symptoms include increased diarrhea, hematochezia, abdominal pain, fever, and malaise. Diagnosis is made endoscopically with biopsies. Differential diagnosis includes infection and undiagnosed Crohn’s disease. The etiology of pouchitis is unknown. Some believe pouchitis results from fecal stasis within the pouch, but emptying studies are not confirmatory. Antibiotics (metronidazole ± ciprofloxacin) are the mainstays of therapy, and most patients will respond rapidly to either oral preparations or enemas.32,33 Some patients develop chronic pouchitis that necessitates ongoing suppressive antibiotic therapy. Salicylate and corticosteroid enemas have also been used with some success. Reintroduction of normal flora by ingestion of probiotics and/or an elemental diet have been suggested as a possible treatment in refractory cases. Occasionally, pouch excision is necessary to control the symptoms of chronic pouchitis.
Many anorectal procedures can be performed with local anesthetic alone. Intravenous sedation is often provided to calm the patient. Injection of 0.5% lidocaine (short acting) and 0.25% bupivacaine (long acting) into the perianal skin, sphincter, and area around the pudendal nerves usually provides an adequate block. The addition of dilute epinephrine decreases bleeding and prolongs the anesthetic effect.
Epidural, spinal, and caudal anesthetics can be used for anorectal procedures and transanal resections. In patients with severe medical comorbidity, regional anesthesia may occasionally be used for laparotomy and colectomy. Postoperative epidural anesthesia provides excellent pain relief and improves pulmonary function.
General anesthesia is required for the vast majority of intra-abdominal procedures. Patients should undergo a thorough preoperative cardiovascular evaluation. In patients with significant comorbid disease, an anesthesia consultation may be appropriate.
Most abdominal colectomies can be performed in the supine position. Anterior resection and APR require lithotomy positioning to facilitate the pelvic dissection and mobilization of the splenic flexure. Adequate padding should be provided for the patient’s sacrum, and care should be taken to avoid stirrup pressure on the peroneal nerves.
Anorectal procedures may be performed in lithotomy or in the prone jackknife position. Some surgeons prefer the prone jackknife position because exposure may be better, especially for anterior lesions. Distal posterior lesions can usually be accessed from either position, but more proximal posterior lesions are better accessed in the prone position.
The rationale for bowel preparation is that decreasing the bacterial load in the colon and rectum will decrease the incidence of postoperative infection. Mechanical bowel preparation uses cathartics to rid the colon of solid stool the night before surgery.28,29 The most commonly used regimens include polyethylene glycol (PEG) solutions or magnesium citrate. PEG solutions require patients to drink a large volume of fluid and may cause bloating and nausea. Magnesium citrate solutions are generally better tolerated but are more likely to cause fluid and electrolyte abnormalities. Both are equally efficacious in bowel cleansing. Preparatory formulations have been recently introduced in tablet form in an attempt to improve tolerance. However, these methods of bowel cleansing require ingestion of 40 or more tablets with water over several hours. To date, these formulations have not been proven to be superior to the more traditional products.34 Antibiotic prophylaxis also is recommended. The addition of oral antibiotics to the preoperative mechanical bowel preparation has been thought to decrease postoperative infection by further decreasing the bacterial load of the colon. However, the regimens used (neomycin, erythromycin, or metronidazole) frequently caused gastrointestinal upset that interfered with the mechanical preparation, and many surgeons have abandoned oral antibiotic prophylaxis. Nevertheless, a recent analysis of the Surgical Care Improvement Project-1 (SCIP-1) suggests that oral antibiotics do reduce postoperative wound infection, especially if a mechanical bowel preparation is not used.35
Prospective randomized trials are needed to better understand the role of oral antibiotic prophylaxis in colorectal surgery. In contrast, longstanding, convincing data support the efficacy of parenteral antibiotic prophylaxis at the time of surgery. Broad-spectrum parenteral antibiotic(s) with activity against aerobic and anaerobic enteric pathogens should be administered just prior to the skin incision and redosed as needed depending on the length of the operation. There is no proven benefit to using antibiotics postoperatively after an uncomplicated colectomy.
Despite widespread use of mechanical bowel preparation, the necessity of bowel cleansing prior to colectomy has been questioned. European surgeons in particular have advocated abandoning this practice. Arguments against mechanical bowel preparation include dehydration and electrolyte abnormalities that often result from bowel cleansing, as well as the risk of spillage of liquid stool left over from the “prep.” Arguments in favor of mechanical bowel preparation included easier manipulation of an “empty” colon (especially in minimally invasive procedures) and avoidance of a “stool column” above an anastomosis, especially in the pelvis. Interestingly, a recent meta-analysis of 14 randomized controlled trials suggested that mechanical bowel preparation does not prevent surgical site infection and should be abandoned in clinical practice.36
Ureteral stents may be useful for identifying the ureters intraoperatively and are placed via cystoscopy after the induction of general anesthesia and removed at the end of the operation. Stents can be invaluable during reoperative pelvic surgery or when there is significant retroperitoneal inflammation (such as complicated diverticulitis), as well as in obese patients. Lighted stents may be helpful in laparoscopic and robotic resections. Patients often have transient hematuria postoperatively, but major complications are rare.
Patients with complex colorectal disease often benefit from a multidisciplinary approach to their care. Patients with pelvic floor disorders (especially incontinence) often require evaluation by both a colorectal surgeon and a urologist or urogynecologist. Preoperative evaluation of cancer patients by a medical oncologist and/or radiation oncologist is crucial for planning either neoadjuvant or adjuvant therapy. Intraoperatively, complex pelvic resections often require the involvement of not only a colorectal surgeon but also a urologist, gynecologic oncologist, neurosurgeon, and/or plastic surgeon. Radiation oncologists should be involved in the operation if brachytherapy catheters are to be placed for intracavitary radiation or if intraoperative radiation therapy is planned. Rarely, psychiatric disorders may manifest as colorectal problems (especially functional disorders and chronic pain), and involvement of a psychiatrist or psychologist may be beneficial.
INFLAMMATORY BOWEL DISEASE
Inflammatory bowel disease includes ulcerative colitis, Crohn’s disease, and indeterminate colitis. Ulcerative colitis occurs in 8 to 15 people per 100,000 in the United States and Northern Europe. The incidence is considerably lower in Asia, Africa, and South America, and among the nonwhite population in the United States. Ulcerative colitis incidence peaks during the third decade of life and again in the seventh decade of life. The incidence of Crohn’s disease is slightly lower, 1 to 5 people per 100,000. Crohn’s disease also affects Northern European and white populations disproportionately. Crohn’s disease has a similar bimodal incidence, with most cases occurring between ages 15 to 30 years and ages 55 to 60 years. In 15% of patients with inflammatory bowel disease, differentiation between ulcerative colitis and Crohn’s colitis is impossible; these patients are classified as having indeterminate colitis.
Many different etiologies for inflammatory bowel disease have been proposed, but none are proven. The consistent geographic differences in incidence suggest an environmental factor such as diet or infection. Alcohol and oral contraceptive use have also been implicated. Smoking has been implicated in the etiology and exacerbation of Crohn’s disease in particular. Family history may play a role because 10% to 30% of patients with inflammatory bowel disease report a family member with the same disease.37,38 Other theories focus on an autoimmune mechanism and/or a defect in the intestinal immune system. While there is general agreement that interaction among the immune system, the mucosal barrier of the gut, and a variety of infectious agents is involved in the pathogenesis of inflammatory bowel disease, the mechanism(s) by which these interactions produce disease is poorly understood. Bacteria, such as Mycobacterium paratuberculosis and Listeria monocytogenes, and viruses, such as paramyxovirus and measles virus, have been suggested as etiologic agents in Crohn’s disease. A defect in the gut mucosal barrier, which increases exposure to intraluminal bacteria, toxins, or proinflammatory substances, also has been suggested. Finally, as noted earlier, an autoimmune mechanism has been postulated. Although there is no clear evidence linking an immunologic disorder to inflammatory bowel disease, the similarity of many of the extraintestinal manifestations to rheumatologic disorders has made this theory attractive. Regardless of the underlying cause of either ulcerative colitis or Crohn’s disease, both disorders are characterized by intestinal inflammation, and medical therapy is largely based on reducing inflammation.
Although ulcerative colitis and Crohn’s colitis share many pathologic and clinical similarities, these conditions may be differentiated in 85% of patients. Ulcerative colitis is a mucosal process in which the colonic mucosa and submucosa are infiltrated with inflammatory cells. The mucosa may be atrophic, and crypt abscesses are common. Endoscopically, the mucosa is frequently friable and may possess multiple inflammatory pseudopolyps. In long-standing ulcerative colitis, the colon may be foreshortened and the mucosa replaced by scar. In quiescent ulcerative colitis, the colonic mucosa may appear normal both endoscopically and microscopically. Ulcerative colitis may affect the rectum (proctitis), rectum and sigmoid colon (proctosigmoiditis), rectum and left colon (left-sided colitis), or the rectum and entire colon (pancolitis). Ulcerative colitis does not involve the small intestine, but the terminal ileum may demonstrate inflammatory changes (“backwash ileitis”). A key feature of ulcerative colitis is the continuous involvement of the rectum and colon; rectal sparing or skip lesions suggest a diagnosis of Crohn’s disease. Symptoms are related to the degree of mucosal inflammation and the extent of colitis. Patients typically complain of bloody diarrhea and crampy abdominal pain. Proctitis may produce tenesmus. Severe abdominal pain and fever raise the concern of fulminant colitis or toxic megacolon. Physical findings are nonspecific and range from minimal abdominal tenderness and distention to frank peritonitis. In the nonemergent setting, the diagnosis is typically made by colonoscopy and mucosal biopsy.
In contrast to ulcerative colitis, Crohn’s disease is a transmural inflammatory process that can affect any part of the gastrointestinal tract from mouth to anus. Mucosal ulcerations, an inflammatory cell infiltrate, and noncaseating granulomas are characteristic pathologic findings. Chronic inflammation may ultimately result in fibrosis, strictures, and fistulas in either the colon or small intestine. The endoscopic appearance of Crohn’s colitis is characterized by deep serpiginous ulcers and a “cobblestone” appearance. Skip lesions and rectal sparing are common. Symptoms of Crohn’s disease depend on the severity of inflammation and/or fibrosis and the location of inflammation in the gastrointestinal tract. Acute inflammation may produce diarrhea, crampy abdominal pain, and fever. Strictures may produce symptoms of obstruction. Weight loss is common, both because of obstruction and from protein loss. Perianal Crohn’s disease may present with pain, swelling, and drainage from fistulas or abscesses. Physical findings are also related to the site and severity of disease.
In 15% of patients with colitis from inflammatory bowel disease, differentiation of ulcerative colitis from Crohn’s colitis is impossible either grossly or microscopically (indeterminate colitis). These patients typically present with symptoms similar to ulcerative colitis. Endoscopic and pathologic findings usually include features common to both diseases. Increasingly, serologic markers have been employed to differentiate ulcerative colitis from Crohn’s disease. The anti-Saccharomyces cerevisiae antibody (ASCA) and perinuclear anticytoplasmic antibody (pANCA) may be useful in differentiating these two processes but require prospective study.39
Further differential diagnoses include infectious colitides, especially Campylobacter jejuni, Entamoeba histolytica, C. difficile, Neisseria gonorrhoeae, Salmonella, and Shigella species.
The liver is a common site of extracolonic disease in inflammatory bowel disease. Fatty infiltration of the liver is present in 40% to 50% of patients, and cirrhosis is found in 2% to 5%. Fatty infiltration may be reversed by medical or surgical treatment of colonic disease, but cirrhosis is irreversible. Primary sclerosing cholangitis is a progressive disease characterized by intra- and extrahepatic bile duct strictures. Forty percent to 60% of patients with primary sclerosing cholangitis have ulcerative colitis. Colectomy will not reverse this disease, and the only effective therapy is liver transplantation.40 Pericholangitis is also associated with inflammatory bowel disease and may be diagnosed with a liver biopsy. Bile duct carcinoma is a rare complication of long-standing inflammatory bowel disease. Patients who develop bile duct carcinoma in the presence of inflammatory bowel disease are, on average, 20 years younger than other patients with bile duct carcinoma.
Arthritis also is a common extracolonic manifestation of inflammatory bowel disease, and the incidence is 20 times greater than in the general population. Arthritis usually improves with treatment of the colonic disease. Sacroiliitis and ankylosing spondylitis are associated with inflammatory bowel disease, although the relationship is poorly understood. Medical and surgical treatment of the colonic disease does not impact symptoms.
Erythema nodosum is seen in 5% to 15% of patients with inflammatory bowel disease and usually coincides with clinical disease activity. Women are affected three to four times more frequently than men. The characteristic lesions are raised, red, and predominantly on the lower legs. Pyoderma gangrenosum is an uncommon but serious condition that occurs almost exclusively in patients with inflammatory bowel disease. The lesion begins as an erythematous plaque, papule, or bleb, usually located on the pretibial region of the leg and occasionally near a stoma. The lesions progress and ulcerate, leading to a painful, necrotic wound. Pyoderma gangrenosum may respond to resection of the affected bowel in some patients. In others, this disorder is unaffected by treatment of the underlying bowel disease.
Up to 10% of patients with inflammatory bowel disease will develop ocular lesions. These include uveitis, iritis, episcleritis, and conjunctivitis. They usually develop during an acute exacerbation of the inflammatory bowel disease. The etiology is unknown.
Medical therapy for inflammatory bowel disease focuses on decreasing inflammation and alleviating symptoms, and many of the agents used are the same for both ulcerative colitis and Crohn’s disease. In general, mild to moderate flares may be treated in the outpatient setting. More severe signs and symptoms mandate hospitalization. Pancolitis generally requires more aggressive therapy than limited disease. Because ulcerative proctitis and proctosigmoiditis are limited to the distal large intestine, topical therapy with salicylate and/or corticosteroid suppositories and enemas can be extremely effective. Systemic therapy is rarely required in these patients.
Sulfasalazine (Azulfidine), 5-acetyl salicylic acid (5-ASA), and related compounds are first-line agents in the medical treatment of mild to moderate inflammatory bowel disease. These compounds decrease inflammation by inhibition of cyclooxygenase and 5-lipoxygenase in the gut mucosa. They require direct contact with affected mucosa for efficacy. Multiple preparations are available for administration to different sites in the small intestine and colon (sulfasalazine, mesalamine [Pentasa, Asacol, Rowasa]).
Antibiotics are often used to decrease the intraluminal bacterial load in Crohn’s disease. Metronidazole has been reported to improve Crohn’s colitis and perianal disease, but the evidence is weak. Fluoroquinolones may also be effective in some cases. In the absence of fulminant colitis or toxic megacolon, antibiotics are not used to treat ulcerative colitis.
Corticosteroids (either oral or parenteral) are a key component of treatment for an acute exacerbation of either ulcerative colitis or Crohn’s disease. Corticosteroids are nonspecific inhibitors of the immune system, and 75% to 90% of patients will improve with the administration of these drugs. However, corticosteroids have a number of serious side effects, and use of these agents should be limited to the shortest course possible. In addition, corticosteroids should be used judiciously in children because of the potential adverse effect on growth. Failure to wean corticosteroids is a relative indication for surgery.
Because of the systemic effects of corticosteroids, an effort has been made to develop drugs that act locally and have limited systemic absorption. Agents such as budesonide, beclomethasone dipropionate, and tixocortol pivalate undergo rapid hepatic degradation that significantly decreases systemic toxicity. Budesonide is available as an oral preparation. Corticosteroid enemas provide effective local therapy for proctitis and proctosigmoiditis and have fewer side effects than systemic corticosteroids.
Azathioprine and 6-mercatopurine (6-MP) are antimetabolite drugs that interfere with nucleic acid synthesis and thus decrease proliferation of inflammatory cells. These agents are useful for treating ulcerative colitis and Crohn’s disease in patients who have failed salicylate therapy or who are dependent on, or refractory to, corticosteroids. It is important to note, however, that the onset of action of these drugs takes 6 to 12 weeks, and concomitant use of corticosteroids almost always is required.37
Cyclosporine is an immunosuppressive agent that interferes with T-lymphocyte function. While cyclosporine is not routinely used to treat inflammatory bowel disease, up to 80% of patients with an acute flare of ulcerative colitis will improve with its use. However, the majority of these patients will ultimately require colectomy. Cyclosporine is also occasionally used to treat exacerbations of Crohn’s disease, and approximately two-thirds of patients will note some improvement. Improvement is generally apparent within 2 weeks of beginning cyclosporine therapy. Long-term use of cyclosporine is limited by its significant toxicities (e.g., nephrotoxicity, hirsutism, gum hypertrophy).
Methotrexate is a folate antagonist that also has been used to treat inflammatory bowel disease. Although the efficacy of this agent is unproven, there are reports that more than 50% of patients will improve with administration of this drug.41
In an effort to improve treatment for steroid-refractory inflammatory bowel disease, a new class of agents has been developed based on inhibition of tumor necrosis factor alpha (TNF-α). Intravenous infusion of these agents decreases inflammation systemically. Infliximab is a monoclonal antibody directed against TNF-α and was the first biologic agent used to treat Crohn’s disease. More than 50% of patients with moderate to severe Crohn’s disease will improve with infliximab therapy.42 This agent also has been useful in treating patients with perianal Crohn’s disease. Recurrence is common, however; and many patients require infusions on a bimonthly basis. Newer anti-TNF-α agents, such as adalimumab and certolizumab pegol, also show promise in treating Crohn’s disease.43
The use of anti-TNF-α agents for the treatment of ulcerative colitis has been less well studied, but there are reports of efficacy in this setting, and adalimumab has been approved for treatment of steroid-refractory ulcerative colitis in Europe.44,45 Interestingly, anti-TNF-α therapy may also be beneficial in treating the extraintestinal manifestations of inflammatory bowel disease in some patients.40
Patients with inflammatory bowel disease are often malnourished. Abdominal pain and obstructive symptoms may decrease oral intake. Diarrhea can cause significant protein loss. Ongoing inflammation produces a catabolic physiologic state. Parenteral nutrition should be strongly considered early in the course of therapy for either Crohn’s disease or ulcerative colitis. The nutritional status of the patient also should be considered when planning operative intervention, and nutritional parameters such as serum albumin, prealbumin, and transferrin should be assessed. In extremely malnourished patients, especially those who are also being treated with corticosteroids, creation of a stoma is often safer than a primary anastomosis.
Ulcerative colitis is a dynamic disease characterized by remissions and exacerbations. The clinical spectrum ranges from an inactive or quiescent phase to low-grade active disease to fulminant disease. The onset of ulcerative colitis may be insidious, with minimal bloody stools, or the onset can be abrupt, with severe diarrhea and bleeding, tenesmus, abdominal pain, and fever. The severity of symptoms depends on the degree and extent of inflammation. Although anemia is common, massive hemorrhage is rare. Physical findings are often nonspecific.
The diagnosis of ulcerative colitis is almost always made endoscopically. Because the rectum is invariably involved, proctoscopy may be adequate to establish the diagnosis. The earliest manifestation is mucosal edema, which results in a loss of the normal vascular pattern. In more advanced disease, characteristic findings include mucosal friability and ulceration. Pus and mucus may also be present. While mucosal biopsy is often diagnostic in the chronic phase of ulcerative colitis, biopsy in the acute phase will often reveal only nonspecific inflammation. Evaluation with colonoscopy or barium enema during an acute flare is contraindicated because of the risk of perforation.
Barium enema has been used to diagnose chronic ulcerative colitis and to determine the extent of disease. However, this modality is less sensitive than colonoscopy and may not detect early disease. In long-standing ulcerative colitis, the colon is foreshortened and lacks haustral markings (“lead pipe” colon). Because the inflammation in ulcerative colitis is purely mucosal, strictures are highly uncommon. Any stricture diagnosed in a patient with ulcerative colitis must be presumed to be malignant until proven otherwise.
Indications for surgery in ulcerative colitis may be emergent or elective. Emergency surgery is required for patients with massive life-threatening hemorrhage, toxic megacolon, or fulminant colitis who fail to respond rapidly to medical therapy. Patients with signs and symptoms of fulminant colitis should be treated aggressively with bowel rest, hydration, broad-spectrum antibiotics, and parenteral corticosteroids. Colonoscopy and barium enema are contraindicated, and antidiarrheal agents should be avoided. Deterioration in clinical condition or failure to improve within 24 to 48 hours mandates surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree