KEY POINTS
Historically, non-small cell cancer (NSCLC) subtypes were considered to be a uniform group based on limited understanding of the distinct clinical behaviors of the subtypes as well as the fact that there were few treatment options available. With increasing understanding of the molecular biology underlying these tumor subtypes, however, the approach to diagnosis and management and the terminology used in describing these tumors are evolving rapidly. In particular, the evaluation and management of adenocarcinoma of the lung has shifted dramatically and firm establishment of NSCLC cell type prior to chemotherapy for advanced stage lung cancer is essential.
A multidisciplinary approach to evaluation of NSCLC, with standardized criteria and terminology for diagnosis in cytologic and small biopsy specimens, and routine molecular testing for known mutations, such as EGFR mutations and EML4-ALK fusion oncogenes is now recommended for the evaluation and management of lung nodules due to major advances in targeted therapy. Adequate tissue acquisition at the time of diagnostic workup is critical and facilitates patient care while minimizing the number of procedures to which the patient is subjected.
The terms bronchioloalveolar carcinoma and mixed subtype adenocarcinoma have been eliminated from the classification of lung adenocarcinoma as a result of increased understanding of important clinical, radiologic, pathologic, and genetic differences between mucinous and nonmucinous adenocarcinomas, The new classification system delineated a stepwise pathologic progression, from AAH to invasive adenocarcinoma based on the predominant histologic growth patterns.
Lung cancer continues to be a highly lethal and extremely common cancer, with 5-year survival of 16%. Lung cancer incidence is second only to the incidence of prostate cancer in men and breast cancer in women. Squamous cell carcinoma and adenocarcinoma of the lung are the most common subtypes and are rarely found in the absence of a smoking history. Nonsmokers who live with smokers have a 24% increased risk of lung cancer compared to nonsmokers who do not live with smokers.
Navigational bronchoscopy is a valuable new tool that can be used to obtain tissue diagnosis for intraparenchymal lesions or small, peripherally located lesions that have historically been difficult to biopsy with transbronchial or transthoracic approaches. It is also a useful tool for tattooing the lung lesion for subsequent operative resection and for placement of fiducial markers for stereotactic body radiation. This technique should become part of the surgeon’s armamentarium for the diagnosis and treatment of lung cancer.
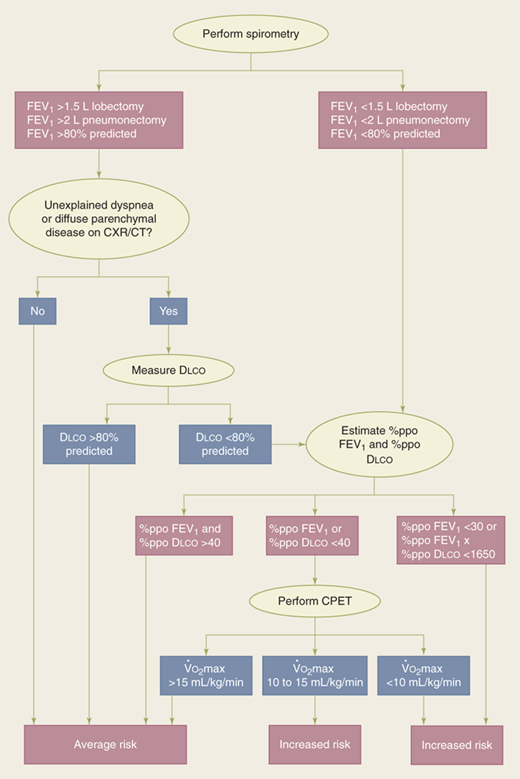
Impaired exchange of carbon monoxide is associated with a significant increase in the risk of postoperative pulmonary complications, independent of the patient’s smoking history. In patients undergoing pulmonary resection, the risk of any pulmonary complication increases by 42% for every 10% decline in the percent carbon monoxide diffusion capacity (%Dlco), and this measure may be a useful parameter in risk stratification of patients for surgery.
Maximum oxygen consumption (V.o2 max) values provide important additional information in those patients with severely impaired Dlco and forced expiratory volume in 1 second. Values of <10 mL/kg per minute generally prohibit any major pulmonary resection, because the mortality in patients with these levels is 26% compared with only 8.3% in patients whose is ≥10 mL/kg per minute; values of >15 mL/kg per minute generally indicate the patient’s ability to tolerate pneumonectomy.
The assessment of patient risk before thoracic resection is based on clinical judgment and data.
Tumor ablative strategies are viable alternatives to surgical resection for early stage lung cancer in inoperable patients. While premature, ablative techniques may ultimately be shown to have efficacy equivalent to lobectomy for the primary treatment of very small peripheral early-stage lung cancers and become primary therapy, even in operable patients. Multidisciplinary collaboration between thoracic surgery, interventional radiology/pulmonology, and radiation oncology is required to ensure that development of these ablative techniques occurs through properly designed and well-controlled prospective studies and will ensure that patients receive the best available therapy, regardless of whether it is surgical resection or ablative therapy.
Increasing evidence suggests a significant role for gastroesophageal reflux disease in the pathogenesis of chronic lung diseases such as bronchiectasis and idiopathic pulmonary fibrosis, and it may also contribute to bronchiolitis obliterans syndrome in lung transplant patients.
Treatment of pulmonary aspergilloma is individualized. Asymptomatic patients can be observed without any additional therapy. Similarly, mild hemoptysis, which is not life-threatening, can be managed with medical therapy, including antifungals and cough suppressant. Amphotericin B is the drug of choice, although voriconazole has recently been used for treatment of aspergillosis, with fewer side effects and equivalent efficacy. Massive hemoptysis had traditionally been an indication for urgent or emergent operative intervention. However, with the advancement of endovascular techniques, bronchial artery embolization in select centers with experience in these techniques has been effective.
In patients with malignant pleural effusion, poor expansion of the lung (because of entrapment by tumor or adhesions) generally predicts a poor result with pleurodesis and is the primary indication for placement of indwelling pleural catheters. These catheters have dramatically changed the management of end-stage cancer treatment because they substantially shorten the amount of time patients spend in the hospital during their final weeks of life.
TRACHEA
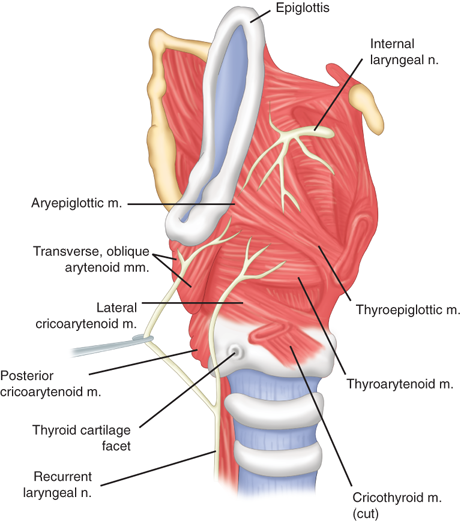
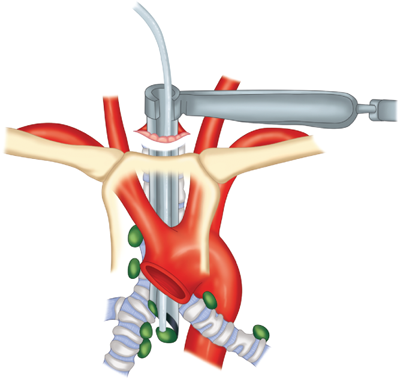
The trachea is composed of cartilaginous and membranous portions, beginning with the cricoid cartilage, the first complete cartilaginous ring of the airway. The cricoid cartilage consists of an anterior arch and a posterior broad-based plate. Articulating with the posterior cricoid plate are the arytenoid cartilages. The vocal cords originate from the arytenoid cartilages and then attach to the thyroid cartilage. The subglottic space, the narrowest part of the trachea with an internal diameter of approximately 2 cm, begins at the inferior surface of the vocal cords and extends to the first tracheal ring. The remainder of the distal trachea is 10.0 to 13.0 cm long, consists of 18 to 22 rings, and has an internal diameter of 2.3 cm (Fig. 19-1).1 Bronchoscopically, the tracheal rings are visible as C-shaped hyaline cartilaginous structures that provide rigidity to the anterior and lateral tracheal walls. The open ends of the C-rings are connected by the trachealis smooth muscle and encased in a dense band of connective tissue called perichondrium. The first tracheal ring is attached directly to the cricoid cartilage; there are approximately two rings for every 1 cm of tracheal length.
The tracheal blood supply, which includes the inferior thyroid, subclavian, supreme intercostal, internal thoracic, innominate, and superior and middle bronchial arteries, enters the airway near the junction of the membranous and cartilaginous portions (Fig. 19-2). Each arterial branch supplies a segment of 1.0 to 2.0 cm, thereby limiting circumferential mobilization to that same distance. The vessels are interconnected along the lateral surface of the trachea by an important longitudinal vascular anastomosis that feeds transverse segmental vessels to the soft tissues between the cartilages.
Tracheal injury can result from a variety of causes, including inhalation of smoke or toxic fumes, aspiration of liquids or solid objects, endotracheal intubation, blunt and penetrating trauma, and iatrogenic injury during operative procedures. Early diagnosis is critical to avoid subsequent complications, including respiratory infection and tracheal stenosis. Management of smoke or toxic fume inhalation and liquid aspiration is commonly supportive; use of antibiotics, respiratory support, and airway clearance with flexible bronchoscopy is dictated by the patient’s condition. In rare circumstances, extracorporeal membrane oxygenation is required if there is associated injury to the more distal airways and lung parenchyma.
Despite ubiquitous use of high-volume–low-pressure cuffs, overinflation of the endotracheal cuff is the most common cause of injury secondary to endotracheal intubation. High cuff pressures can cause ischemia of the contiguous airway wall in as short as 4 hours. Prolonged overinflation can lead to scarring and stenosis; full-thickness injury can result in fistulae between the innominate artery anteriorly and the esophagus posteriorly. Avoidance requires careful cuff management to keep pressures as low as possible; in circumstances of prolonged ventilatory support and high airway pressure, cuff pressure monitoring (to maintain pressures <20 mmHg) is advisable.
Historically, clinically significant tracheal stenosis after tracheostomy occurred in 3% to 12% of cases, with severe stenosis in 1% to 2%.2 With the use of low-pressure cuffs, the estimated incidence has decreased to 4.9 cases per million patients per year.3 Intubation-related risk factors include: prolonged intubation; high tracheostomy through the first tracheal ring or cricothyroid membrane; transverse rather than vertical incision on the trachea; oversized tracheostomy tube; prior tracheostomy or intubation; and traumatic intubation. Stenosis is also more common in older patients, in females, after radiation, or after excessive corticosteroid therapy, and in the setting of concomitant diseases such as autoimmune disorders, severe reflux disease, or obstructive sleep apnea and the setting of severe respiratory failure. However, even a properly placed tracheostomy can lead to tracheal stenosis because of scarring and local injury. Mild ulceration and stenosis are frequently seen after tracheostomy removal. Use of the smallest tracheostomy tube possible, rapid downsizing, and a vertical tracheal incision minimize the risk for posttracheostomy stenosis.
Stridor and dyspnea on exertion are the primary symptoms of tracheal stenosis. In the setting of postintubation injury, a significant portion of the cartilaginous structural support to the airway is destroyed by regional ischemic necrosis; during healing, a web-like fibrous growth develops and narrows the airway (Fig. 19-3). In contrast, stenosis caused by tracheostomy is most commonly due to an excess of granulation tissue formation around the tracheal stoma site. Time to onset of symptoms after extubation or tracheostomy decannulation usually ranges from 2 to 12 weeks, but symptoms can appear immediately or as long as 1 to 2 years later. Frequently, patients are misdiagnosed as having asthma or bronchitis, and treatment for such illnesses can persist for some time before the correct diagnosis is discovered. Generally, symptom intensity is related to the degree of stenosis and to the patient’s underlying pulmonary disease.
Figure 19-3.
Diagram of the principal postintubation lesions. A. A circumferential lesion at the cuff site after the use of an endotracheal tube. B. Potential lesions after the use of tracheostomy tubes. Anterolateral stenosis can be seen at the stomal level. Circumferential stenosis can be seen at the cuff level (lower than with an endotracheal tube). The segment in between is often inflamed and malacotic. C. Damage to the subglottic larynx. D. Tracheoesophageal fistula occurring at the level of the tracheostomy cuff; circumferential damage is usual at this level. E. Tracheoinnominate artery fistula. (Adapted with permission from Grillo H. Surgical treatment of postintubation tracheal injuries. J Thorac Cardiovasc Surg. 1979;78:860. Copyright Elsevier)
A comprehensive bronchoscopic evaluation is critical in the initial phase of evaluation. Stenosis length, location, distance between the vocal cords and proximal stenosis, and distance from the distal aspect to the major carina must be documented. In patients with severe stenosis and respiratory compromise, rigid bronchoscopy can be used to dilate the stenosis; this provides immediate relief of the airway obstruction and facilitates thorough evaluation of the stenosis. Rarely, if ever, is tracheostomy necessary.
Most intubation injuries are located in the upper third of the trachea and can be accessed for resection through a collar incision. Resection typically involves 2 to 4 cm of trachea for benign stenosis. It is critical to fully resect all inflamed and scarred tissue. However, a primary anastomosis can still be performed without undue tension, even if up to one half of the trachea requires resection.2 Ideally, the patient is extubated in the operating room or shortly thereafter. For patients in whom tracheal resection is not possible, such as patients with significant comorbidities or with an excessively long stenosis, endotracheal stenting, typically silicone T-tubes, can provide palliation. Wire mesh stents should not be used, given their known propensity to erode through the wall of the airway. Balloon dilation, laser ablation, and tracheoplasty have also been described, although the efficacy is marginal.
Tracheal replacement is evolving as an option for management of tracheal stenosis as bioengineering techniques for decellularizing donor trachea have been developed. This removes all antigens against which the recipient immune system might react and enables use of the donor trachea scaffolding without risk of rejection. Following decellularization, the donor tracheal scaffolding is seeded with recipient chondrocytes, to restore tracheal rigidity, and with recipient epithelial cells, to recreate the inner epithelial lining. Several case reports of successful allogeneic tracheal transplantation have been published, but the technique continues to be limited to a few highly specialized centers. This is due, in part, to the scarcity of donor trachea and the need for tissue bioengineering expertise. Current efforts are focused on creation of biosynthetic scaffolding that can be used instead of donor trachea. This would substantially increase the availability of the tracheal replacement material and enable widespread use of the technique.
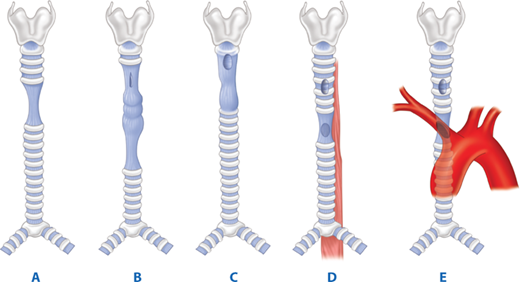
Tracheoinnominate artery fistula has two main causes: low placement of a tracheostomy and hyperinflation of the tracheal cuff. Tracheostomy placement should be through the second to fourth tracheal rings without reference to the location of the sternal notch. When placed below the fourth tracheal ring, the inner curve of the tracheostomy cannula will be positioned to exert pressure on the posterior aspect of the innominate artery, leading to arterial erosion. Similarly, the tracheal cuff, when hyperinflated, will cause ischemic injury to the anterior airway and subsequent erosion into the artery. Most cuff-induced fistulas will develop within 2 weeks after placement of the tracheostomy.
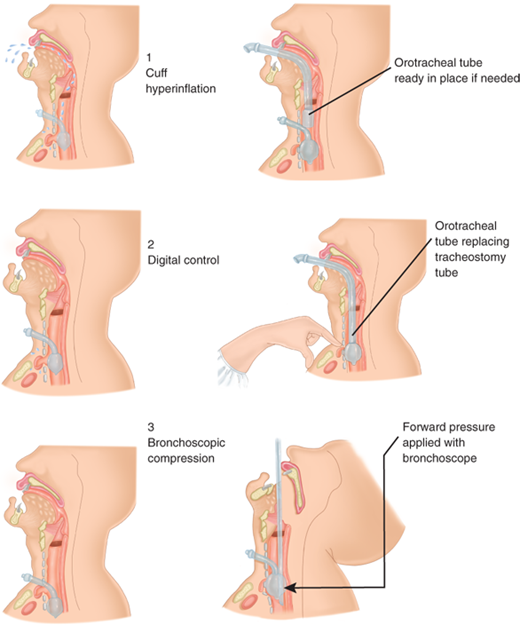
Clinically, tracheoinnominate artery fistulas present with bleeding. A premonitory hemorrhage often occurs and, although it is usually not massive, must not be ignored or simply attributed to general airway irritation or wound bleeding. With significant bleeding, the tracheostomy cuff can be hyperinflated to temporarily occlude the arterial injury. If such an effort is unsuccessful, the tracheostomy incision should be immediately opened widely and a finger inserted to compress the artery against the manubrium (Fig. 19-4). The patient can then be orally intubated, and the airway suctioned free of blood. Emergent surgical resection of the involved segment of artery is performed, usually without reconstruction.
Tracheoesophageal fistulas (TEFs) occur primarily in patients receiving prolonged mechanical ventilatory support concomitant with an indwelling nasogastric tube.4 Cuff compression of the membranous trachea against the nasogastric tube leads to airway and esophageal injury and fistula development. Clinically, airway suctioning reveals saliva, gastric contents, or tube feedings. Gastric insufflation, secondary to positive pressure ventilation, can occur. Bronchoscopy is diagnostic; with the bronchoscope inserted, the endotracheal tube is withdrawn and the fistula at the cuff site is exposed. Alternatively, esophagoscopy demonstrates the cuff of the endotracheal tube in the esophagus.
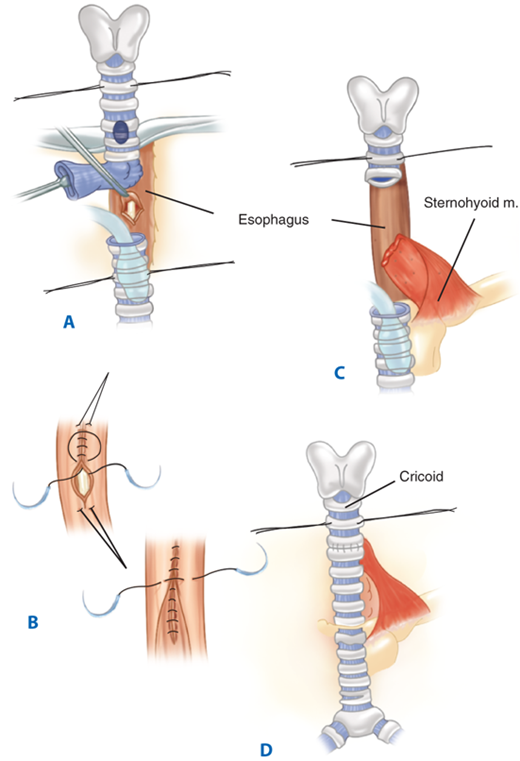
Treatment, first and foremost, requires removing tubes from the esophagus and weaning the patient from the ventilator. The cuff of the endotracheal tube should be placed below the fistula, avoiding overinflation. To minimize aspiration, a gastrostomy tube should be placed for gastric decompression (to prevent reflux) and a jejunostomy tube for feeding. If aspiration persists, esophageal diversion with cervical esophagostomy can be performed. Once weaned from the ventilator, tracheal resection and primary anastomosis, repair of the esophageal defect, and interposition of a muscle flap between the trachea and esophagus can be performed (Fig. 19-5).5
Figure 19-5.
Single-stage operation for closure of a tracheoesophageal fistula and tracheal resection. A. The fistula is divided and the trachea is transected below the level of damage. B. The fistula is closed on the tracheal side in a single layer and the esophageal side in a double layer. The damaged trachea segment is resected. C. View of completed tracheal anastomosis. m. = muscle.
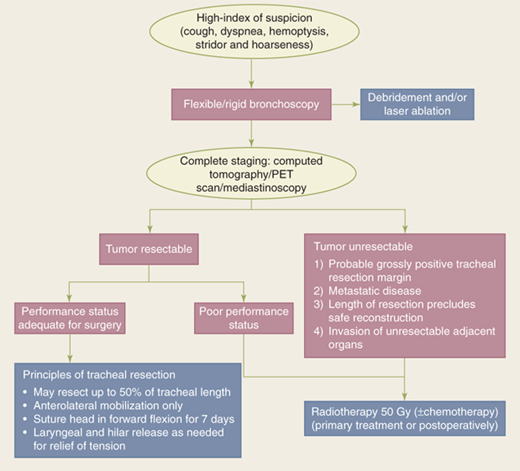
Although extremely rare, the most common primary tracheal neoplasms are squamous cell carcinomas (related to smoking) and adenoid cystic carcinomas. Clinically, tracheal tumors present with cough, dyspnea, hemoptysis, stridor, or symptoms of invasion of contiguous structures (such as the recurrent laryngeal nerve or the esophagus). The most common radiologic finding of tracheal malignancy is tracheal stenosis, but is found in only 50% of cases. With tumors other than squamous cell carcinomas, symptoms may persist for months because of slow tumor growth rates. Stage of presentation is advanced, with approximately 50% of patients presenting with stage IV disease. Five-year survival for all tracheal neoplasms is 40% but falls to 15% for those with stage IV disease.6
Squamous cell carcinomas often present with regional lymph node metastases and are frequently unresectable at presentation. Their biologic behavior is similar to that of squamous cell carcinoma of the lung. Adenoid cystic carcinomas, a type of salivary gland tumor, are generally slow-growing, spread submucosally, and tend to infiltrate along nerve sheaths and within the tracheal wall. Although indolent in nature, adenoid cystic carcinomas are malignant and can spread to regional lymph nodes, lung, and bone. Squamous cell carcinoma and adenoid cystic carcinomas represent approximately 65% of all tracheal neoplasms. The remaining 35% is comprised of small cell carcinomas, mucoepidermoid carcinomas, adenocarcinomas, lymphomas, and others.7
Evaluation and treatment of patients with tracheal tumors should include neck and chest computed tomography (CT) and rigid bronchoscopy. Rigid bronchoscopy permits general assessment of the airway and tumor; it also allows debridement or laser ablation of the tumor to provide relief of dyspnea. If the tumor is judged to be completely resectable, primary resection and anastomosis is the treatment of choice for these tumors (Fig. 19-6). Up to 50% of the length of the trachea can be resected with primary anastomosis. In most tracheal resections, anterolateral tracheal mobilization and suturing of the chin to the sternum for 7 days are done routinely. Use of laryngeal and hilar release is determined at the time of surgery, based on the surgeon’s judgment of the degree of tension present. For longer resections, specialized maneuvers are necessary such as laryngeal release and right hilar release to minimize tension on the anastomosis.
Postoperative mortality, which occurs in up to 10% of patients, is associated with the length of tracheal resection, use of laryngeal release, the type of resection, and the histologic type of the cancer. Factors associated with improved long-term survival include complete resection and use of radiation as adjuvant therapy in the setting of incomplete resection.8 Due to their radiosensitivity, radiotherapy is frequently given postoperatively after resection of both adenoid cystic carcinomas and squamous cell carcinomas.9 A dose of 50 Gy or greater is usual. Nodal positivity does not seem to be associated with worse survival. Survival at 5 and 10 years is much better for adenoid cystic (73% and 57%, respectively) than for tracheal cancers (47% and 36%, respectively; P < .05). For patients with unresectable tumors, radiation may be given as the primary therapy to improve local control, but is rarely curative. For recurrent airway compromise, stenting or laser therapies should be considered part of the treatment algorithm.
LUNG
The segmental bronchial and vascular anatomy of the lungs allows subsegmental and segmental resections, if the clinical situation requires it or if lung tissue can be preserved10 (Fig. 19-7). Note the continuity of the pulmonary parenchyma between adjacent segments of each lobe.
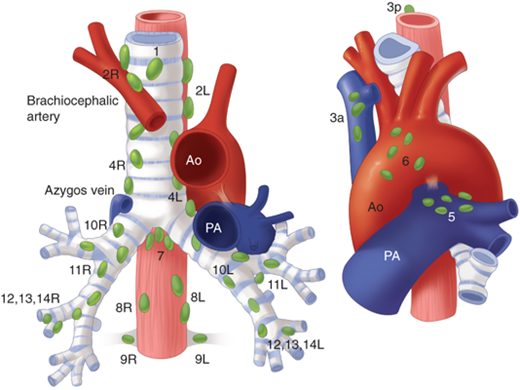
Lymph nodes that drain the lungs are divided into two groups according to the tumor-node-metastasis (TNM) staging system for lung cancer: the pulmonary lymph nodes (N1) and the mediastinal nodes (N2) (Fig. 19-8).
The N1 lymph nodes constitute the following: (a) intrapulmonary or segmental nodes that lie at points of division of segmental bronchi or in the bifurcations of the pulmonary artery; (b) lobar nodes that lie along the upper, middle, and lower lobe bronchi; (c) interlobar nodes located in the angles formed by the main bronchi bifurcating into the lobar bronchi; and (d) hilar nodes along the main bronchi. The interlobar lymph nodes lie in the depths of the interlobar fissure on each side and constitute a lymphatic sump for each lung, referred to as the lymphatic sump of Borrie; all of the pulmonary lobes of the corresponding lung drain into this group of nodes (Fig. 19-9). On the right, the nodes of the lymphatic sump lie around the bronchus intermedius (bounded above by the right upper lobe bronchus and below by the middle lobe and superior segmental bronchi). On the left, the lymphatic sump is confined to the interlobar fissure, with the lymph nodes in the angle between the lingular and lower lobe bronchi and in apposition to the pulmonary artery branches.
The N2 lymph nodes consist of four main groups. (a) The anterior mediastinal nodes are located in association with the upper surface of the pericardium, the phrenic nerves, the ligamentum arteriosum, and the left innominate vein. (b) The posterior mediastinal group includes paraesophageal lymph nodes within the inferior pulmonary ligament and, more superiorly, between the esophagus and trachea near the arch of the azygos vein. (c) The tracheobronchial lymph nodes are made up of three subgroups that are located near the bifurcation of the trachea. These include the subcarinal nodes, which lie in the obtuse angle between the trachea and each main stem bronchus, and the nodes that lay anterior to the lower end of the trachea. (d) Paratracheal lymph nodes are located in proximity to the trachea in the superior mediastinum. Those on the right side form a chain with the tracheobronchial nodes inferiorly and with some of the deep cervical nodes above (scalene lymph nodes).
Lymphatic drainage to the mediastinal lymph nodes from the right lung is ipsilateral, except for occasional bilateral drainage to the superior mediastinum. In contrast, in the left lung, particularly the left lower lobe, lymphatic drainage occurs with equal frequency to ipsilateral and contralateral superior mediastinal nodes.
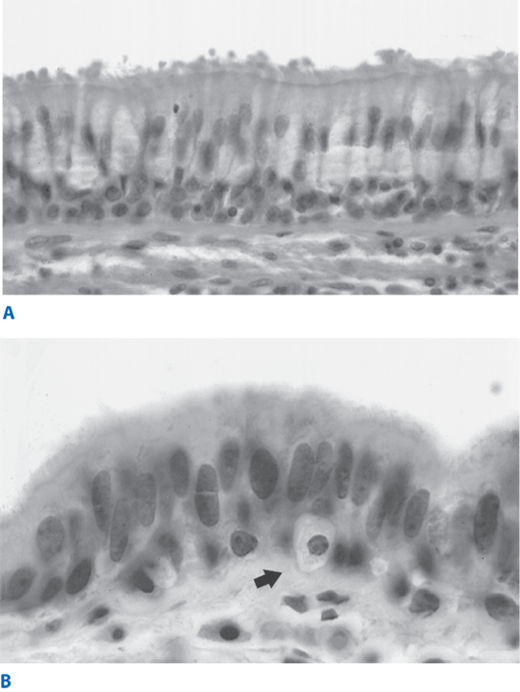
The lung can be conveniently viewed as two linked components: the tracheobronchial tree (or conducting airways component) and the alveolar spaces (or gas exchange component). The tracheobronchial tree consists of approximately 23 airway divisions to the level of the alveoli. It includes the main bronchi, lobar bronchi, segmental bronchi (to designated bronchopulmonary segments), and terminal bronchioles (i.e., the smallest airways still lined by bronchial epithelium and without alveoli). The tracheobronchial tree is normally lined by pseudostratified ciliated columnar cells and mucous (or goblet) cells, which both derive from basal cells (Fig. 19-10). Ciliated cells predominate. Goblet cells, which release mucus, can significantly increase in number in acute bronchial injury, such as exposure to cigarette smoke. The normal bronchial epithelium also contains bronchial submucosal glands, which are mixed salivary-type glands containing mucous cells, serous cells, and neuroendocrine cells called Kulchitsky cells, which are also found within the surface epithelium. The bronchial submucosal glands can give rise to salivary gland–type tumors, including mucoepidermoid carcinomas and adenoid cystic carcinomas.
Two cell types, called type I and type II pneumocytes, make up the alveolar epithelium. Type I pneumocytes comprise 40% of the total number of alveolar epithelial cells, but cover 95% of the surface area of the alveolar wall. These cells are not capable of regeneration because they have no mitotic potential. Type II pneumocytes cover only 3% of the alveolar surface, but comprise 60% of the alveolar epithelial cells. In addition, clusters of neuroendocrine cells are seen in the alveolar spaces.
The term “precancerous” does not mean that an inevitable progression to invasive carcinoma will occur, but such lesions, particularly those with high-grade dysplasia,11,12 do constitute a clear marker for potential development of invasive cancer. Three precancerous lesions of the respiratory tract are currently recognized.
Squamous dysplasia and carcinoma in situ. Cigarette smoke can induce a transformation of the tracheobronchial pseudostratified epithelium to metaplastic squamous mucosa, with subsequent evolution to dysplasia as cellular abnormalities accumulate. Dysplastic changes include altered cellular polarity and increased cell size, number of cell layers, nuclear-to-cytoplasmic ratio, and number of mitoses. Gradations are considered mild, moderate, or severe. Carcinoma in situ represents carcinoma still confined by the basement membrane.
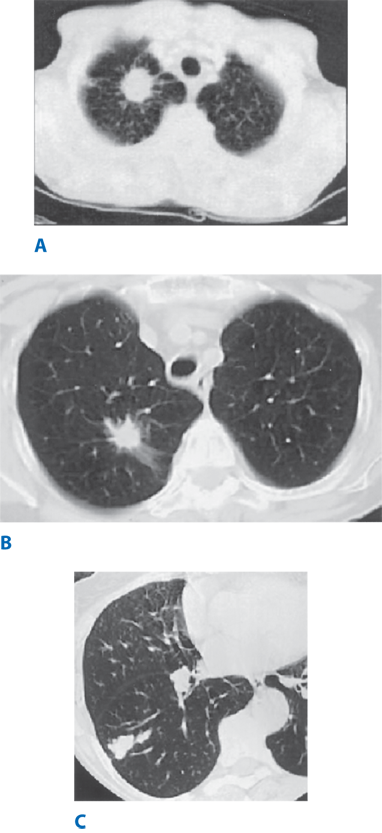
Atypical adenomatous hyperplasia (AAH). AAH is a lesion smaller than 5.0 mm, comprising epithelial cells lining the alveoli that are similar to type II pneumocytes. Histologically, AAH is similar to adenocarcinoma in situ; it represents the beginning stage of a stepwise evolution to adenocarcinoma in situ and then to adenocarcinoma. With the availability of thin-section CT, it is possible to detect preinvasive adenocarcinoma lesions as early as AAH. These lesions can be multiple, are typically small (5 mm or less), and have a ground-glass appearance.
Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. This rare lesion represents a diffuse proliferation of neuroendocrine cells, but without invasion of the basement membrane. It can exist as a diffuse increase in the number of single neuroendocrine cells, or as small lesions less than 5.0 mm in diameter. Lesions over 5.0 mm in size or that breach the basement membrane are carcinoid tumors.
The pathologic diagnosis of lung cancer is currently based on light microscopic criteria and is broadly divided into two main groups: non–small cell lung carcinoma and neuroendocrine tumors.13 Immunohistochemical staining and electron microscopy are used as adjuncts in diagnosis, particularly in the assessment of potential neuroendocrine tumors.
The term non–small cell lung carcinoma (NSCLC) includes many tumor cell types, including large cell, squamous cell, and adenocarcinoma. Historically, these subtypes were considered to be a uniform group based on limited understanding of the distinct clinical behaviors of the subtypes as well as the fact that there were few treatment options available. With increasing understanding of the molecular biology underlying these tumor subtypes, however, the approach to diagnosis and management and the terminology used in describing these tumors are evolving rapidly.
The incidence of adenocarcinoma has increased over the last several decades, and it is now the most common lung cancer, accounting for 30% of lung cancers in male smokers and 40% of lung cancers in female smokers. Adenocarcinoma is the histologic subtype for 80% and 60% of lung cancers in nonsmoking females and males, respectively. It occurs more frequently in females than in males. It is the most frequent histologic subtype in women, patients who are under 45 years of age, and Asian populations.14
Increasing understanding of lung adenocarcinoma, such as important clinical, radiologic, pathologic, and genetic differences between mucinous and nonmucinous adenocarcinomas, prompted multiple changes in the classification system in 2011.15 Based on consensus, the international working group proposed a multidisciplinary approach, with standardized criteria and terminology for diagnosis in cytologic and small biopsy specimens, and routine molecular testing for known mutations, such as EGFR and KRAS mutations (Table 19-1). The new classification system delineated a stepwise pathlogic progression, from AAH to invasive adenocarcinoma based on the predominant histologic growth patterns; the terms bronchioloalveolar carcinoma and mixed subtype adenocarcinoma were eliminated in favor of more biologically driven classification (Table 19-2).
| INVASIVE MUCINOUS ADENOCARCINOMA (FORMERLY MUCINOUS BAC) | NONMUCINOUS AIS/MIA/LPA (FORMERLY NONMUCINOUS BAC) | |
|---|---|---|
| Female | 49/84 (58%)52,120,121,123 | 101/140 (72%)52,120,121,123 |
| Smoker | 39/87 (45%)52,120,121,123,124 | 75/164 (46%)52,120,121,122,124 |
| Radiographic appearance | Majority consolidation; air bronchogram125 | Majority ground-glass attenuation23,56,58,103,129,130,131,132,133,134 |
| Frequent multifocal and multilobar presentation56,125,126,127,128 | ||
| Cell type | Mucin-filled, columnar, and/or goblet50,51,52,125,135 | Type II pneumocyte and/or Clara cell50,51,52,125,135 |
| Phenotype | ||
| CK7 | Mostly positive (~88%)a54,55,136,137,138,139 | Positive (~98%)a54,55,136,137,138,139 |
| CK20 | Positive (~54%)a54,55,136,137,138,139 | Negative (~5%)a54,55,136,137,138,139 |
| TTF-1 | Mostly negative (~17%)1 a54,55,120,137,138,139 | Positive (~67%)a54,55,120,137,138,139 |
| Genotype | ||
| KRAS mutation | Frequent (~76%)a55,94,121,127,140,141,142,143,144 | Some (~13%)a55,121,127,140,141,142,143,144 |
| EGFR mutation | Almost none (~3)a55,121,127,140,141,142 | Frequent (~45%)a55,121,127,140,141,142 |
Preinvasive lesions Atypical adenomatous hyperplasia Adenocarcinoma in situ (≤3 cm formerly BAC) Nonmucinous Mucinous Mixed mucinous/nonmucinous |
Minimally invasive adenocarcinoma (≤3 cm lepidic predominant tumor with ≤5 mm invasion) Nonmucinous Mucinous Mixed mucinous/nonmucinous |
Invasive adenocarcinoma Lepidic predominant (formerly nonmucinous BAC pattern, with >5 mm invasion) Acinar predominant Papillary predominant Micropapillary predominant Solid predominant with mucin production |
Variants of invasive adenocarcinoma Invasive mucinous adenocarcinoma (formerly mucinous BAC) Colloid Fetal (low and high grade) Enteric |
Adenocarcinoma in situ (AIS). AISs are small (≤3 cm) solitary adenocarcinomas that have pure lepidic growth; lepidic growth is characterized by tumor growth within the alveolar spaces. These lesions are not invasive into the stroma, vascular system, or pleura and do not have papillary or micropapillary patterns or intra-alveolar tumor cells. They are very rarely mucinous, consisting of type II pneumocytes or Clara cells. These patients are expected to have 100% disease-specific survival with complete surgical resection. On CT scan, AIS can appear as a pure ground-glass neoplasm, but occasionally will present as part of a solid or part-solid nodule. Mucinous AIS is more likely to appear solid or to have the appearance of consolidation. As with AAH, the lesions can be single or multiple; the ground-glass changes in AIS, however, tend to have a higher attenuation compared to AAH.
Minimally invasive adenocarcinoma (MIA). In the same size solitary lesion, if less than 5 mm of invasion are noted within a predominantly lepidic growth pattern, the lesion is termed minimally invasive adenocarcinoma (MIA) to indicate a patient group with near 100% survival when the lesion is completely resected. This differentiates patients with AIS, but recognizes the fact that the presence of invasion becomes prognostically significant when the size of the invasive component reaches 5 mm or greater in size.16 If multiple areas of microscopic invasion are found within the lepidic growth, the size of the largest invasive area, measured in the largest dimension, is used; this area must be ≤5 mm to be considered MIA. As with AIS, MIA is very rarely mucinous. The invasive component histologically is acinar, papillary, micropapillary, and/or solid and shows tumor cells infiltrating into the surrounding myofibroblastic stroma. On CT scan, the appearance of MIA is often a part-solid nodule (≤5 mm) with a predominant ground-glass component, but can be highly variable.
Lepidic predominant adenocarcinoma (LPA). If lymphovascular invasion, pleural invasion, tumor necrosis, or more than 5 mm of invasion are noted in a lesion that has lepidic growth as its predominant component, MIA is excluded and the lesion is called lepidic predominant adenocarcinoma (LPA), and the size of the invasive component is recorded for the T stage.
Invasive adenocarcinoma. The new classification system now recommends classifying invasive adenocarcinoma by the most predominant subtype after histologic evaluation of the resection specimen. To determine the predominant subtype, histologic sections are evaluated and the patterns are determined, in 5% increments, throughout the specimen. This semiquantitative method encourages the viewer to identify and quantify all patterns present, rather than focusing on a single pattern. In the pathology report, the tumor is classified by the predominant pattern, with percentages of the subtypes also reported (Fig. 19-11).Subtypes include:
Lepidic predominant
Acinar predominant
Papillary predominant
Micropapillary predominant
Solid predominant
Adenocarcinoma is often peripherally located and frequently discovered incidentally on routine chest radiographs, unlike squamous cell cancers. When symptoms occur, they are due to pleural or chest wall invasion (pleuritic or chest wall pain) or pleural seeding with malignant pleural effusion. Invasive adenocarcinoma is usually solid by CT scan, but can also be part-solid and even a ground-glass nodule. Occasionally, a lobar ground-glass opacification may be present, which is often associated with significant respiratory compromise and can be mistaken for lobar pneumonia. Bubble-like or cystic lucencies on CT scan in small (≤2 cm) adenocarcinomas or extensive associated ground-glass components correlate with slow growth and well-differentiated tumors and a more favorable prognosis. Intratumoral air bronchograms are usually indicative of well-differentiated tumor, whereas spiculations that are coarse and thick (≥2 mm) portend vascular invasion and nodal metastasis and are associated with decreased survival following complete surgical resection. Pleural retraction is also a poor prognostic indicator.
Additional histologic variants include colloid adenocarcinoma (formerly mucinous cystadenocarcinoma), fetal adenocarcinoma, and enteric adenocarcinoma. Clear cell and signet ring cell types are no longer considered to be distinct subtypes as they are found in association with most of the five dominant histologic patterns (lepidic, acinar, papillary, micropapillary, and solid). However, they are still notable, as they can signal clinically relevant molecular changes, such as the presence of the EML4-ALK fusion gene in solid tumors with signet ring features.
Figure 19-11.
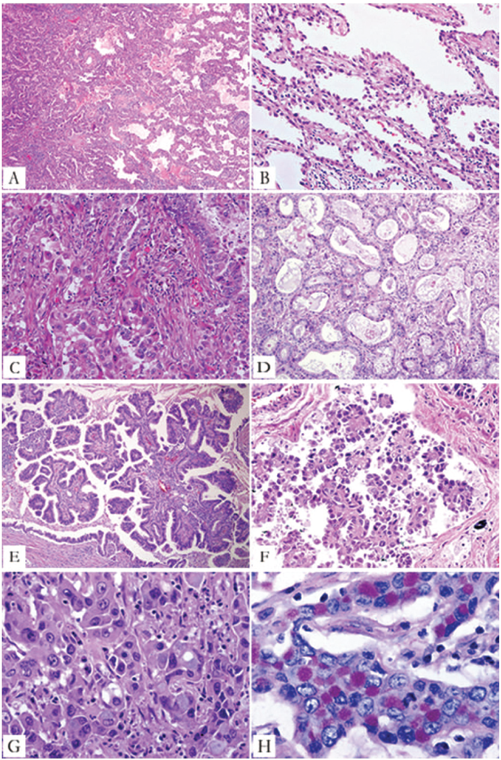
Major histologic patterns of invasive adenocarcinoma. A. Lepidic predominant pattern with mostly lepidic growth (right) and a smaller area of invasive acinar adenocarcinoma (left). B. Lepidic pattern consists of a proliferation type II pneumocytes and Clara cells along the surface alveolar walls. C. Area of invasive acinar adenocarcinoma (same tumor as in A and B). D. Acinar adenocarcinoma consists of round to oval-shaped malignant glands invading a fibrous stroma. E. Papillary adenocarcinoma consists of malignant cuboidal to columnar tumor cells growing on the surface of fibrovascular cores. F. Micropapillary adenocarcinoma consists of small papillary clusters of glandular cells growing within this airspace, most of which do not show fibrovascular cores. G. Solid adenocarcinoma with mucin consisting of sheets of tumor cells with abundant cytoplasm and mostly vesicular nuclei with several conspicuous nucleoli. No acinar, papillary, or lepidic patterns are seen, but multiple cells have intracytoplasmic basophilic globules that suggest intracytoplasmic mucin. H. Solid adenocarcinoma with mucin. Numerous intracytoplasmic droplets of mucin are highlighted with this diastase-periodic acid Schiff stain. (Reproduced with permission from Travis W, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244.)
Representing 30% to 40% of lung cancers, squamous cell carcinoma is the most frequent cancer in men and highly correlated with cigarette smoking. They arise primarily in the main, lobar, or first segmental bronchi, which are collectively referred to as the central airways. Symptoms of airway irritation or obstruction are common, and include cough, hemoptysis, wheezing (due to high-grade airway obstruction), dyspnea (due to bronchial obstruction with or without postobstructive atelectasis), and pneumonia (caused by airway obstruction with secretion retention and atelectasis).
Occasionally a more peripherally based squamous cell carcinoma will develop in a tuberculosis scar or in the wall of a bronchiectatic cavity. Histologically, cells develop a pattern of clusters with intracellular bridges and keratin pearls. Central necrosis is frequent and may lead to the radiographic findings of a cavity (possibly with an air-fluid level). Such cavities may become infected, with resultant abscess formation.
Large cell carcinoma accounts for 10% to 20% of lung cancers and may be located centrally or peripherally. These tumors have cell diameters of 30 to 50 μm, which are often admixed with various other malignant cell types. Large cell carcinoma can be confused with a large cell variant of neuroendocrine carcinoma, but can be differentiated by special immunohistochemical stains.
Salivary-type submucosal bronchial glands throughout the tracheobronchial tree can give rise to tumors that are histologically identical to those seen in the salivary glands. The two most common are adenoid cystic carcinoma and mucoepidermoid carcinoma. Both tumors occur centrally due to their site of origin. Adenoid cystic carcinoma is a slow-growing tumor that is locally and systemically invasive, growing submucosally and infiltrating along perineural sheaths. Mucoepidermoid carcinoma consists of squamous and mucous cells and is graded as low or high grade, depending on the mitotic rate and degree of necrosis.
Neuroendocrine lung tumors are classified into neuroendocrine hyperplasia and three separate grades of neuroendocrine carcinoma (NEC). Immunohistochemical staining for neuroendocrine markers (including chromogranins, synaptophysin, CD57, and neuron-specific enolase) is essential to accurately diagnose most tumors.17
Grade I NEC (classic or typical carcinoid) is a low-grade NEC; 80% arise in the epithelium of the central airways. It occurs primarily in younger patients. Because of the central location, it classically presents with hemoptysis, with or without airway obstruction and pneumonia. Histologically, tumor cells are arranged in cords and clusters with a rich vascular stroma. This vascularity can lead to life-threatening hemorrhage with even simple bronchoscopic biopsy maneuvers. Regional lymph node metastases are seen in 15% of patients, but rarely spread systemically or cause death.
Grade II NECs (atypical carcinoid) have a much higher malignant potential and, unlike grade I NEC, are etiologically linked to cigarette smoking and are more likely to be peripherally located. Histologic findings may include areas of necrosis, nuclear pleomorphism, and higher mitotic rates. Lymph node metastases are found in 30% to 50% of patients. At diagnosis, 25% of patients already have remote metastases.
Grade III NEC large cell–type tumors occur primarily in heavy smokers and in the mid to peripheral lung fields. They are often large with central necrosis and a high mitotic rate. Their neuroendocrine nature is revealed by positive immunohistochemical staining for at least one neuroendocrine marker.
Grade IV NEC (small cell lung carcinoma [SCLC]) is the most malignant NEC and accounts for 25% of all lung cancers; these NECs often have early, widespread metastases. These cancers also arise primarily in the central airways. As with squamous cell cancers, symptoms include cough, hemoptysis, wheezing (due to high-grade airway obstruction), dyspnea (due to bronchial obstruction with or without postobstructive atelectasis), and pneumonia (caused by airway obstruction with secretion retention and atelectasis). Evaluation includes expert pathology review and comprehensive evaluation for metastatic disease. Three groups of grade IV NEC are recognized: pure small cell carcinoma (sometimes referred to as oat cell carcinoma), small cell carcinoma with a large cell component, and combined (mixed) tumors.
Grade IV NECs consist of smaller cells (diameter 10 to 20 μm) with little cytoplasm and very dark nuclei; they can be difficult to distinguish from lymphoproliferative lesions and atypical carcinoid tumors. Histologically, a high mitotic rate with easily visualized multiple mitoses and areas of extensive necrosis are characteristic. Importantly, very small bronchoscopic biopsies can distinguish NSCLC from SCLC, but crush artifact may make NSCLC appear similar to SCLC. If uncertainty exists, special immunohistochemical stains or rebiopsy (or both) will be necessary. These tumors are the leading producer of paraneoplastic syndromes.
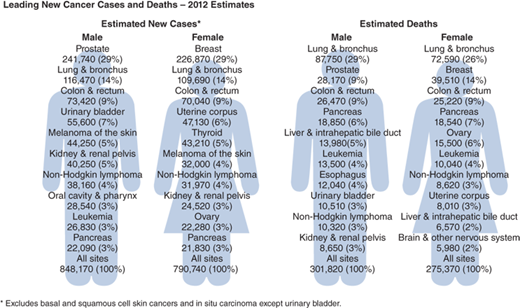
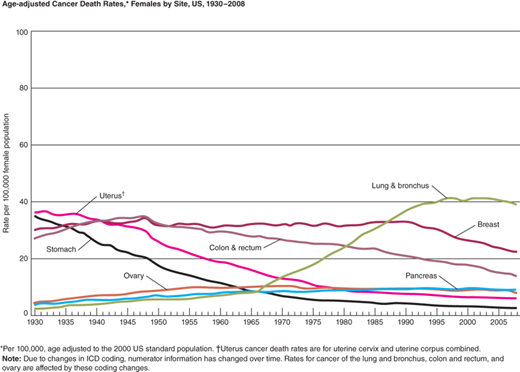
Lung cancer is the leading cancer killer and second most frequently diagnosed cancer in the United States, accounting for nearly 28% of all cancer deaths—more than cancers of the breast, prostate, ovary, and colon and rectum combined (Fig. 19-12). In 2008, it was estimated that 1 in 13 men and 1 in 16 women would develop lung cancer in their lifetime. The overall 5-year survival for all patients with lung cancer is 16%, making lung cancer the most lethal of the leading four cancers (Fig. 19-13A, B) It is encouraging, however, that the average annual death rate declined by 2.8% per year for men and 1.1% per year for women from 2005 to 2009.18 Unfortunately, most patients are still diagnosed at an advanced stage of disease, so therapy is rarely curative.
Figure 19-12.
Leading new cancer cases and deaths: 2012 estimates. *Excludes basal and squamous cell skin cancers and in situ carcinomas except urinary bladder. (Modified with permission from John Wiley and Sons: Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2012;62:10. © 2012 American Cancer Society, Inc.)
Figure 19-13.
Age-adjusted cancer death rates. A. Males by site, United States, 1930 to 2008. B. Females by site, United States, 1930 to 2008. *Per 100,000, age adjusted to the 2000 U.S. standard population. (Modified with permission from John Wiley and Sons: Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2012;62:10. © 2012 American Cancer Society, Inc.)
Prognostic markers for lung cancer survival include female sex (5-year survival of 18.3% for women vs. 13.8% for men), younger age (5-year survival of 22.8% for those <45 years vs. 13.7% for those >65 years), and white race (5-year survival of 16.1% for whites vs. 12.2% for blacks). When access to advanced medical care is unrestricted, as for the military population, the racial difference in survival disappears, suggesting that, at least in part, differences in survival may be explained by less access to advanced medical care and later diagnosis.19
Cigarette smoking was implicated as a causal factor in approximately 75% of all lung cancers worldwide in 2007. According to the U.S. Surgeon General’s report in 2004, 90% of lung cancers in men and nearly 80% in women can be attributed to cigarette smoking or secondhand cigarette smoke exposure. Two lung cancer types—squamous cell and small cell carcinoma—are extraordinarily rare in the absence of cigarette smoking. The risk of developing lung cancer escalates with the number of cigarettes smoked, the number of years of smoking, and the use of unfiltered cigarettes. Conversely, the risk of lung cancer declines with smoking cessation, but never drops to that of never smokers, regardless of the length of abstinence (Table 19-3).20 Radon exposure accounts for the vast majority of the remaining cancers. Approximately 25% of all lung cancers worldwide and 53% of cancers in women are not related to smoking, and most of them (62%) are adenocarcinomas. Table 19-4 summarizes the existing data regarding the etiology of lung cancer in nonsmokers.21
| RISK FACTOR | RISK ESTIMATE (95% CI) | COMMENTS | REFERENCE |
|---|---|---|---|
| Environmental tobacco smoke | 1.19 (90% CI: 1.04–1.35) 1.21 (1.13–1.30) 1.22 (1.13–1.33) 1.24 (1.18–1.29) | Meta-analysis of 11 U.S. studies of spousal exposure (females only) Meta-analysis of 44 case-control studies worldwide of spousal exposure Meta-analysis of 25 studies worldwide of workplace exposure Meta-analysis of 22 studies worldwide of workplace exposure | 225 226 226 227 |
| Residential radon | 8.4% (3.0%–15.8%) per 100 Bq m3 increase in measured radon 11% (0%–28%) per 100 Bq m3 | Meta-analysis of 13 European studies Meta-analysis of 7 North American studies | 228 229 |
| Cooking oil vapors | 2.12 (1.81–2.47) | Meta-analysis of 7 studies from China and Taiwan (females who never smoked) | 230 |
| Indoor coal and wood burning | 2.66 (1.39–5.07) 1.22 (1.04–1.44) 2.5 (1.5–3.6) | Meta-analysis of 7 studies from China and Taiwan (both sexes) Large case-control study (2861 cases and 3118 controls) from Eastern and Central Europe (both sexes) Large case-control study (1205 cases and 1541 controls) from Canada (significant for women only) | 230 231 232 |
| Genetic factors: family history, CYP1A1 Ile462Val polymorphism, XRCC1 variants | 1.51 (1.11–2.06) 2.99 (1.51–5.91) 2.04 (1.17–3.54) No association No association overall; reduced risk 0.65 (0.46–0.83) with Arg194Trp polymorphism and 0.56 (0.36–0.86) with Arg280His for heavy smokers Increased risk for never smokers 1.3 (1.0–1.8) and decreased risk for heavy smokers 0.5 (0.3–1.0) with Arg299Gln | Meta-analysis of 28 case-control, 17 cohort, and 7 twin studies Meta-analysis of 14 case-control studies of Caucasian never smokers Meta-analysis of 21 case-control studies of Caucasian and Asian never smokers (significant for Caucasians only) Meta-analysis of 13 case-control studies Large case-control study from Europe (2188 cases and 2198 controls) Large case-control study from the United States (1091 cases and 1240 controls) | 233 234 235 236 237 238 |
| Viral factors: HPV 16 and 18 | 10.12 (3.88–26.4) for never smoking women >60 y | Case-control study (141 cases, 60 controls) from Taiwan of never smoking women | 239 |
Nearly 3500 deaths from lung cancer each year are attributable to secondhand (environmental) smoke exposure, which confers an excess risk for lung cancer of 24% when a nonsmoker lives with a smoker.22 Risk is conferred by exposure to any burning tobacco, including cigars. The amount of secondhand exposure from one large cigar is equivalent to the exposure from 21 cigarettes. As with active smoking, risk of developing lung cancer increases with longer duration and higher level of exposure to environmental tobacco.
Over 7000 chemicals have been identified in tobacco smoke, and more than 70 of the compounds are known to be carcinogens. The main chemical carcinogens are polycyclic aromatic hydrocarbons, which are actively or passively inhaled in the tobacco smoke and absorbed; these compounds are activated by specific enzymes and become mutagenic, bind to macromolecules such as deoxyribonucleic acid (DNA), and induce genetic mutations. In treating any patient with a previous smoking history, it is important to remember that there has been field cancerization of the entire aerodigestive tract. The patient’s risk is increased for cancers of the oral cavity, pharynx, larynx, tracheobronchial tree and lung, and esophagus. In examining such patients, a detailed history and physical examination of these organ systems must be performed.
Other causes of lung cancer include exposure to a number of industrial compounds, including asbestos, arsenic, and chromium compounds. In fact, the combination of asbestos and cigarette smoke exposure has a multiplicative effect on risk. Pre-existing lung disease confers an increased risk of lung cancer—up to 13%—for individuals who have never smoked. Patients with chronic obstructive pulmonary disease are at higher risk for lung cancer than would be predicted based on smoking risk alone. Patients with secondary scar formation related to a history of tuberculosis also have a higher risk of primary lung carcinoma. This increase is thought to be related to poor clearance of inhaled carcinogens and/or to the effects of chronic inflammation.
In 2002, the National Lung Screening Trial (NLST) was launched to determine whether screening with CT in high-risk populations would reduce mortality from lung cancer. The study randomized 53,353 eligible patients age 55 to 74 years to either three annual low-dose helical CT scans (LDCT; aka spiral CT) or posteroanterior view chest radiograph. Patients were eligible for the trial if they had a greater than 30 pack-year history of cigarette smoking; had smoked within the past 15 years if a former smoker; had no prior history of lung cancer; had no history of other life-threatening cancers in the prior 5 years; did not have symptoms suggestive of an undiagnosed lung cancer (such as hemoptysis or weight loss); and had not had a chest CT scan in the prior 18 months. Accrual to the study was excellent, and the primary endpoint of a 20% relative reduction in mortality was achieved in 2010. An absolute risk reduction of lung cancer death of four per 1000 individuals screened by LDCT was realized. Interestingly, all-cause mortality was also reduced by nearly 7% in the LDCT group, further emphasizing the impact of lung cancer on the mortality of smokers and former smokers.23 An estimated 320 individuals need to be screened to save one life from lung cancer. Additional considerations require further evaluation before widespread LDCT screening will become reality. First, there was a 7% false-positive rate in this trial. False-positive scans lead to patient anxiety, invasive testing, and potentially morbid procedures to further evaluate the finding. The impact of these issues on patient quality of life and cost-effectiveness has yet to be elucidated. It is also critical that regulatory guidelines for patient eligibility, frequency of screening, interpretation of the scans, processes for further evaluation and management of positive findings, and dose of radiation are well established and well accepted to ensure the generalizability of the results for patients who will be screened in the general medical community rather than in the specialized centers that performed the trial.
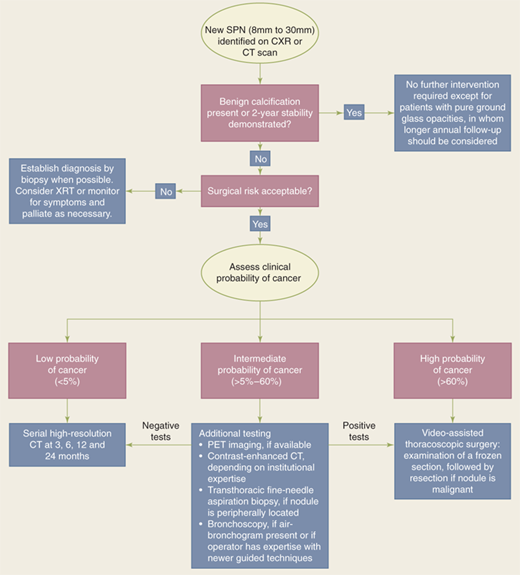
A solitary pulmonary nodule is typically described as a single, well-circumscribed, spherical lesion that is 3 cm or less in diameter and completely surrounded by normal aerated lung parenchyma.24 Lung atelectasis, hilar enlargement, and pleural effusion are absent. The majority are detected incidentally on chest radiographs (CXRs) or CT scans obtained for some other purpose. About 150,000 solitary nodules are found incidentally each year. The clinical significance of such a lesion depends on whether or not it represents a malignancy.
The differential diagnosis of a solitary pulmonary nodule should include a broad variety of congenital, neoplastic, inflammatory, vascular, and traumatic disorders. The probability of cancer in a solitary pulmonary nodule increases if the patient has a history of smoking (50% or higher for smokers compared to 20%–40% in never smokers). It is also more likely to be malignant if it is symptomatic or the patient is older, male, or has had occupational exposures.
Solitary pulmonary nodules were defined by findings on CXR, but with the increased sensitivity of low-dose screening CT, up to 50% of solitary lesions are found to be associated with multiple (one to six) other, usually subcentimeter, nodules. In the Early Lung Cancer Action project, almost 7% of healthy volunteers were found to have between one and three nodules and 25% had up to six nodules. CT scanning is necessary to characterize nodule number, location, size, margin morphology, calcification pattern, and growth rate.25 Spiral (helical) CT allows continuous scanning as the patient is moved through a scanning gantry, allowing the entire thorax to be imaged during a single breath hold (Fig. 19-14). Compared to conventional CT, this provides a superior image quality, because motion artifacts are eliminated, and improves detection of pulmonary nodules and central airway abnormalities.26 The shorter acquisition time of spiral CT also allows for consistent contrast filling of the great vessels, resulting in markedly improved visualization of pathologic states and anatomic variation contiguous to vascular structures. In addition, three-dimensional spiral CT images can be reconstructed for enhanced visualization of spatial anatomic relationships.27 Thin sections (1–2 mm collimation) at 1-cm intervals should be used to evaluate pulmonary parenchyma and peripheral bronchi. If the goal is to find any pulmonary metastases, thin sections at intervals of 5 to 7 mm collimation are recommended. For assessing the trachea and central bronchi, collimation of 3 to 5 mm is recommended. Providing accurate clinical history and data is of paramount importance to obtaining appropriate imaging.
Figure 19-14.
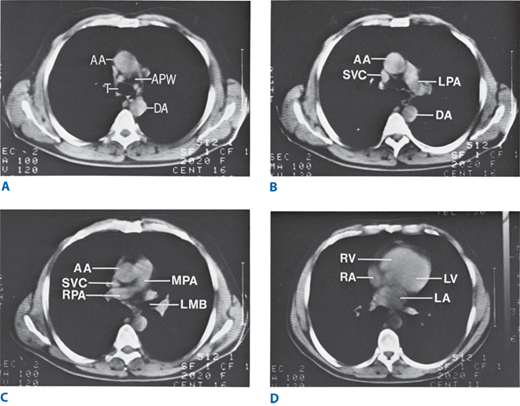
Spiral computed tomography scan showing normal transverse chest anatomy at four levels. A. At the level of the tracheal bifurcation, the aorticopulmonary window can be seen. B. The origin of the left pulmonary artery can be seen at a level 1 cm inferior to A. C. The origin and course of the right pulmonary artery can be seen at this next most cephalad level. The left upper lobe bronchus can be seen at its origin from the left main bronchus. D. Cardiac chambers and pulmonary veins are seen in the lower thorax. AA = ascending aorta; APW = aorticopulmonary window; DA = descending aorta; LA = left ventricle; LMB = left main bronchus; LPA = left pulmonary artery; MPA = main pulmonary artery; RA = right atrium; RPA = right pulmonary artery; RV = right ventricle; SVC = superior vena cava; T = trachea.
CT findings characteristic of benign lesions include small size, calcification within the nodule, and stability over time. Four patterns of benign calcification are common: diffuse, solid, central, and laminated or “popcorn.” Granulomatous infections such as tuberculosis can demonstrate the first three patterns, whereas the popcorn pattern is most common in hamartomas. In areas of endemic granulomatous disease, differentiating benign versus malignant can be challenging. Infectious granulomas arising from a variety of organisms account for 70% to 80% of this type of benign solitary nodules; hamartomas are the next most common single cause, accounting for about 10%.
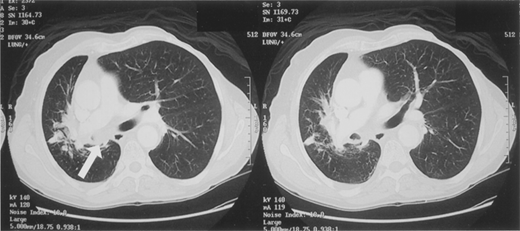
CT findings characteristic of malignancy include growth over time; increasing density on CT scan (40%–50% of partial solid lesions are malignant compared to only 15% of subcentimeter solid or nonsolid nodules); size >3 cm; irregular, lobulated, or spiculated edges; and the finding of the corona radiata sign (consisting of fine linear strands extending 4–5 mm outward and appearing spiculated on radiographs) (Fig. 19-15). Calcification that is stippled, amorphous, or eccentric is usually associated with cancer.
Figure 19-15.
Computed tomography scan images of solitary pulmonary nodules. A. The corona radiata sign demonstrated by a solitary nodule. Multiple fine striations extend perpendicularly from the surface of the nodule like the spokes of a wheel. B. A biopsy-proven adenocarcinoma demonstrating spiculation. C. A lesion with a scalloped border, an indeterminate finding suggesting an intermediate probability for malignancy.
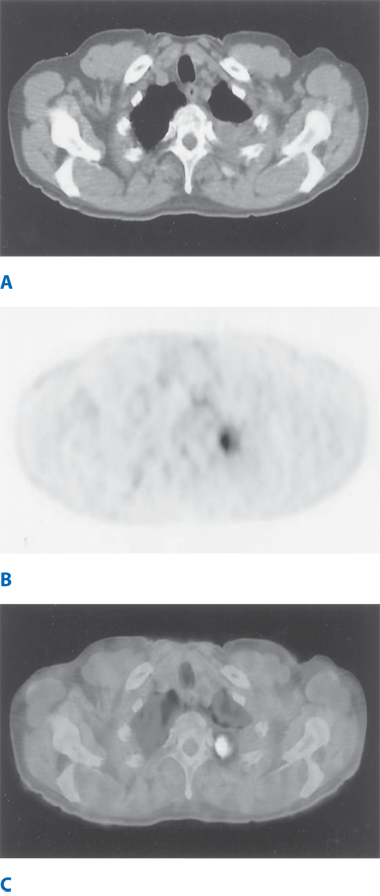
Growth over time is an important characteristic for differentiating benign and malignant lesions. Lung cancers have volume-doubling times from 20 to 400 days; lesions with shorter doubling times are likely due to infection, and longer doubling times suggest benign tumors, but can represent slower-growing lung cancer. Positron emission tomography (PET) scanning can differentiate benign from malignant nodules28; most lung tumors have increased signatures of glucose uptake, as compared with healthy tissues and, thus, glucose metabolism can be measured using radio-labeled 18F-fluorodeoxyglucose (FDG). Meta-analysis estimates 97% sensitivity and 78% specificity for predicting malignancy in a nodule. False-negative results can occur (especially in patients who have AIS, MIA, or LPA, carcinoids, and tumors <1 cm in diameter), as well as false-positive results (because of confusion with other infectious or inflammatory processes).
The cause of a new pulmonary nodule(s) in a patient with a previous malignancy can be difficult to discern.29 Features suggestive of metastatic disease are multiplicity; smooth, round borders on CT scan; and temporal proximity to the original primary lesion. One must always entertain the possibility that a single new lesion is a primary lung cancer. The probability of a new primary cancer vs. metastasis in patients presenting with solitary lesions depends on the type of initial neoplasm. The highest likelihood of a new primary lung cancer is in patients with a history of uterine (74%), bladder (89%), lung (92%), and head and neck (94%) carcinomas.
Surgical resection of pulmonary metastases has a role in properly selected patients.30 The best data regarding outcomes of resection of pulmonary metastases come from the International Registry of Lung Metastases (IRLM). The registry was established in 1991 by 18 thoracic surgery departments in Europe, the United States, and Canada, and included data on 5206 patients. About 88% of patients underwent complete resection. Survival analysis at 5, 10, and 15 years (grouping all primary tumor types) was performed (Table 19-5). Multivariate analysis showed a better prognosis for patients with germ cell tumors, osteosarcomas, a disease-free interval over 36 months, and a single metastasis.31 Depicted in Fig. 19-16, survival after metastasectomy in a variety of cancers is optimal when metastatic disease is resectable, solitary, and identified 36 or more months after initial treatment. When any or all of these optimal characteristics are absent, survival progressively declines.
Figure 19-16.
The actuarial survival after metastasectomy is depicted for patients with various tumor types further categorized into four groups according to resectability, solitary or multiple, the interval between primary resection and metastesectomy, and a combination of factors known in our work and in others, as follows: (1) resectable, solitary, and disease-free interval (DFI) greater than or equal to 36 months; (2) resectable, solitary, or DFI 36+ months; (3) resectable, multiple metastases, and DFI <36 months; and (4) unresectable. (Reprinted with permission from Pastorino, U. (2010). The Development of an International Registry. J Thorac Oncol. 5(6): S196-S197)
The general principles of patient selection for metastasectomy are listed in Table 19-6. The technical aim of pulmonary metastasectomy is complete resection of all macroscopic tumors. In addition, any involved adjacent structures should be resected en bloc (i.e., chest wall, diaphragm, and pericardium). Multiple lesions and/or hilar lesions may require lobectomy. Pneumonectomy is rarely justified or employed.
1. Primary tumor must already be controlled. 2. Patient must be able to tolerate general anesthesia, potential single-lung ventilation, and the planned pulmonary resection. 3. Metastases must be completely resectable based on computed tomographic imaging. 4. There is no evidence of extrapulmonary tumor burden. 5. Alternative superior therapy must not be available. |
Pulmonary metastasectomy can be approached through a thoracotomy or via video-assisted thoracic surgery (VATS) techniques. McCormack and colleagues reported their experience at Memorial Sloan-Kettering in a prospective study of 18 patients who presented with no more than two pulmonary metastatic lesions and underwent VATS resection.32 A thoracotomy was performed during the same operation; if palpation identified any additional lesions, they were resected. The study concluded that the probability that a metastatic lesion will be missed by VATS excision is 56%. Patients in the Memorial study were evaluated before the advent of spiral CT scanning, however, and it remains controversial whether metastasis resection should be performed via VATS. Proponents of VATS argue that the resolution of spiral CT scanning is so superior that prior studies using standard CT scanners are no longer relevant. Indeed, a recent study suggested that only 18% of malignant nodules would be missed using a VATS approach in the current era while another study from the United Kingdom found equivalent outcomes with regard to missed lesions and pulmonary progression comparing open and VATS approaches. To date, no prospective study using spiral CT scan has been performed to resolve this clinical dilemma.
Lung cancer displays one of the most diverse presentation patterns of all human maladies (Table 19-7). The wide range of symptoms and signs is related to (a) histologic features, which often help determine the anatomic site of origin in the lung; (b) the specific tumor location in the lung and its relationship to surrounding structures; (c) biologic features and the production of a variety of paraneoplastic syndromes; and (d) the presence or absence of metastatic disease. Symptoms related to the local intrathoracic effect of the primary tumor can be conveniently divided into two groups: pulmonary and nonpulmonary thoracic.
| CATEGORY | SYMPTOM | CAUSE |
|---|---|---|
| Pulmonary symptoms | Cough | Bronchus irritation or compression |
| Dyspnea | Airway obstruction or compression | |
| Wheezing | >50% airway obstruction | |
| Hemoptysis | Tumor erosion or irritation | |
| Pneumonia | Airway obstruction | |
| Nonpulmonary thoracic symptoms | Pleuritic pain | Parietal pleural irritation or invasion |
| Local chest wall pain | Rib and/or muscle involvement | |
| Radicular chest pain | Intercostal nerve involvement | |
| Pancoast’s syndrome | Stellate ganglion, chest wall, brachial plexus involvement | |
| Hoarseness | Recurrent laryngeal nerve involvement | |
| Swelling of head and arms | Bulky involved mediastinal lymph nodes | |
| Medially based right upper lobe tumor |
Pulmonary symptoms result from the direct effect of the tumor on the bronchus or lung tissue. Symptoms (in order of frequency) include cough (secondary to irritation or compression of a bronchus), dyspnea (usually due to central airway obstruction or compression, with or without atelectasis), wheezing (with narrowing of a central airway of >50%), hemoptysis (typically, blood streaking of mucus that is rarely massive; indicates a central airway location), pneumonia (usually due to airway obstruction by the tumor), and lung abscess (due to necrosis and cavitation, with subsequent infection).
Nonpulmonary thoracic symptoms result from invasion of the primary tumor directly into a contiguous structure (e.g., chest wall, diaphragm, pericardium, phrenic nerve, recurrent laryngeal nerve, superior vena cava, and esophagus), or from mechanical compression of a structure (e.g., esophagus or superior vena cava) by enlarged tumor-bearing lymph nodes.
Peripherally located tumors (often adenocarcinomas) extending through the visceral pleura lead to irritation or growth into the parietal pleura and potentially to continued growth into the chest wall structures. Three types of symptoms, depending on the extent of chest wall involvement, are possible: (a) pleuritic pain, from noninvasive contact of the parietal pleura with inflammatory irritation or direct parietal pleural invasion; (b) localized chest wall pain, from deeper invasion and involvement of the rib and/or intercostal muscles; and (c) radicular pain, from involvement of the intercostal nerve(s). Radicular pain may be mistaken for renal colic in the case of tumors invading the inferoposterior chest wall.
Other specific nonpulmonary thoracic symptoms include:
Pancoast’s syndrome. Tumors originating in the superior sulcus (posterior apex) elicit: apical chest wall and/or shoulder pain (from involvement of the first rib and chest wall); Horner’s syndrome (unilateral enophthalmos, ptosis, miosis, and facial anhidrosis from invasion of the stellate sympathetic ganglion); and radicular arm pain (from invasion of T1, and occasionally C8, brachial plexus nerve roots).
Phrenic nerve palsy. The phrenic nerve traverses the hemithorax along the mediastinum, parallel and posterior to the superior vena cava and anterior to the pulmonary hilum. Tumors at the medial lung surface or anterior hilum can directly invade the nerve; symptoms include shoulder pain (referred), hiccups, and dyspnea with exertion because of diaphragm paralysis. Radiographically, unilateral diaphragm elevation on CXR is present; the diagnosis is confirmed by fluoroscopic examination of the diaphragm with breathing and sniffing (the “sniff” test).
Recurrent laryngeal nerve palsy. Recurrent laryngeal nerve (RLN) involvement most commonly occurs on the left side, given the hilar location of the left RLN as it passes under the aortic arch. Paralysis results from: (a) invasion of the vagus nerve above the aortic arch by a medially based left upper lobe tumor; or (b) direct invasion of the RLN by hilar tumor and/or hilar or aortopulmonary lymph node metastases. Symptoms include voice change, often referred to as hoarseness, but more typically a loss of tone associated with a breathy quality, and coughing, particularly when drinking liquids.
Superior vena cava (SVC) syndrome. As a result of bulky enlargement of involved mediastinal lymph nodes compressing or a medially based right upper lobe tumor invading the SVC, SVC syndrome symptoms include variable degrees of swelling of the head, neck, and arms; headache; and conjunctival edema. It is seen most commonly with NEC grade IV (small cell) lung cancer.
Pericardial tamponade. Pericardial effusions (benign or malignant), associated with increasing levels of dyspnea and/or arrhythmias, and pericardial tamponade occur with direct pericardial invasion. Diagnosis requires a high index of suspicion in the setting of a medially based tumor with symptoms of dyspnea and is confirmed by CT scan or echocardiography.
Back pain. Results from direct invasion of a vertebral body and is often localized and severe. If the neural foramina are involved, radicular pain may also be present.
Other local symptoms. Dysphagia is usually secondary to external esophageal compression by enlarged lymph nodes involved with metastatic disease, usually with lower lobe tumors. Finally, dyspnea, pleural effusion, or referred shoulder pain can result from invasion of the diaphragm by a tumor at the base of a lower lobe.
All lung cancer histologies are capable of producing a variety of paraneoplastic syndromes, most often from systemic release of tumor-derived biologically active materials (Table 19-8). Paraneoplastic syndromes may produce symptoms even before any local symptoms are produced by the primary tumor, thereby aiding in early diagnosis. Their presence does not influence resectability or treatment options. Symptoms often abate with successful treatment; paraneoplastic symptom recurrence may herald tumor recurrence. The majority of such syndromes are associated with grade IV NEC (small cell carcinoma), including many endocrinopathies.
Endocrine Hypercalcemia (ectopic parathyroid hormone) Cushing’s syndrome Syndrome of inappropriate secretion of antidiuretic hormone Carcinoid syndrome Gynecomastia Hypercalcitoninemia Elevated growth hormone level Elevated levels of prolactin, follicle-stimulating hormone, luteinizing hormone Hypoglycemia Hyperthyroidism Neurologic Encephalopathy Subacute cerebellar degeneration Progressive multifocal leukoencephalopathy Peripheral neuropathy Polymyositis Autonomic neuropathy Eaton-Lambert syndrome Optic neuritis Skeletal Clubbing Pulmonary hypertrophic osteoarthropathy Hematologic Anemia Leukemoid reactions Thrombocytosis Thrombocytopenia Eosinophilia Pure red cell aplasia Leukoerythroblastosis Disseminated intravascular coagulation Cutaneous Hyperkeratosis Dermatomyositis Acanthosis nigricans Hyperpigmentation Erythema gyratum repens Hypertrichosis lanuginosa acquista Other Nephrotic syndrome Hypouricemia Secretion of vasoactive intestinal peptide with diarrhea Hyperamylasemia Anorexia or cachexia |
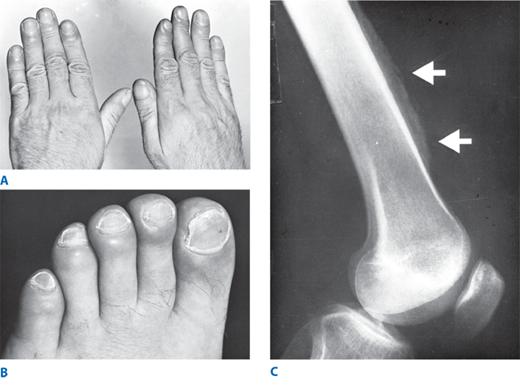
Hypertrophic pulmonary osteoarthropathy (HPO). Often severely debilitating, symptoms of HPO may antedate the diagnosis of cancer by months. Clinically, ankle, feet, forearm, and hand tenderness and swelling are characteristic, resulting from periostitis of the fibula, tibia, radius, metacarpals, and metatarsals. Clubbing of the digits may occur in up to 30% of patients with grade IV NEC (Fig. 19-17). Plain radiographs show periosteal inflammation and elevation, while bone scans demonstrate intense but symmetric uptake in the long bones. Aspirin or nonsteroidal anti-inflammatory agents provide temporary relief; treatment requires successful surgical or medical tumor eradication.
Hypercalcemia. Up to 10% of patients with lung cancer will have hypercalcemia, most often due to metastatic disease. Ectopic parathyroid hormone secretion by the tumor, most often squamous cell carcinoma, is causative in up to 15%, however, and should be suspected if metastatic bone disease is not present. Symptoms of hypercalcemia include lethargy, depressed level of consciousness, nausea, vomiting, and dehydration. Most patients have resectable tumors, and, following complete resection, the calcium level will normalize. Unfortunately, tumor recurrence is extremely common and may manifest as recurrent hypercalcemia.
Hyponatremia. Characterized by confusion, lethargy, and possible seizures, hyponatremia can result from the inappropriate secretion of antidiuretic hormone from the tumor into the systemic circulation (syndrome of inappropriate secretion of antidiuretic hormone [SIADH]) in 10% to 45% of patients with grade IV NEC (small cell). It is diagnosed by the presence of hyponatremia, low serum osmolality, and high urinary sodium and osmolality. Another cause of hyponatremia can be the ectopic secretion of atrial natriuretic peptide (ANP).
Cushing’s syndrome. Autonomous tumor production of an adrenocorticotropic hormone (ACTH)-like molecule leads to rapid serum elevation of ACTH and subsequent severe hypokalemia, metabolic alkalosis, and hyperglycemia. Symptoms are primarily related to the metabolic changes while the physical signs of Cushing’s syndrome (e.g., truncal obesity, buffalo hump, striae) are unusual due to the rapidity of ACTH elevation. Diagnosis is made by demonstrating hypokalemia (<3.0 mmol/L); nonsuppressible elevated plasma cortisol levels that lack the normal diurnal variation; elevated blood ACTH levels; or elevated urinary 17-hydroxycorticosteroids, all of which are not suppressible by administration of exogenous dexamethasone. Immunoreactive ACTH is present in nearly all extracts of SCLC, and a high percentage of patients with SCLC have elevated ACTH levels by radioimmunoassay, yet fewer than 5% have symptoms of Cushing’s syndrome.
Peripheral and central neuropathies. Unlike other paraneoplastic syndromes, which are usually due to ectopic secretion of an active substance, these syndromes are felt to be immune mediated. Cancer cells are thought to secrete antigens normally expressed only by the nervous system, generating antibodies leading either to interference with neurologic function or to immune neurologic destruction. Up to 16% of lung cancer patients have neuromuscular disability, and, of these, half have grade IV NEC (small cell) and 25% have squamous cell carcinomas. In patients with neurologic or muscular symptoms, central nervous system (CNS) metastases must be ruled out with CT or magnetic resonance imaging (MRI) of the head. Other metastatic disease leading to disability must also be excluded.
Lambert-Eaton syndrome. This myasthenia-like syndrome is caused by tumor secretion of immunoglobulin G (IgG) antibodies targeting voltage-gated calcium channels, which causes a neuromuscular conduction defect by decreasing the amount of acetylcholine released from presynaptic sites at the motor end plate. Symptoms, including gait abnormalities from proximal muscle weakness and impaired coordination, may actually precede radiographic evidence of the tumor. Therapy is directed at the primary tumor with resection, radiation, and/or chemotherapy. Many patients have dramatic improvement after successful therapy. For patients with refractory symptoms, treatment consists of guanidine hydrochloride, immunosuppressive agents such as prednisone and azathioprine, and occasionally plasma exchange. Unlike with myasthenia gravis patients, neostigmine is usually ineffective.
Lung cancer metastasizes most commonly to the CNS, vertebral bodies, bone, liver, adrenal glands, lungs, skin, and soft tissues. CNS metastases are present at diagnosis in 10% of patients; another 10% to 15% will develop CNS metastases following diagnosis. Focal symptoms, including headache, nausea, vomiting, seizures, hemiplegia, and dysarthria, are common. Lung cancer is the most common cause of spinal cord compression, either by primary tumor invasion of an intervertebral foramen or direct extension of vertebral metastases. Bony metastases are identified in 25% of all patients with lung cancer. They are primarily lytic and produce pain locally; thus any new and localized skeletal symptoms must be evaluated radiographically. Liver metastases are most often an incidental finding on CT scan. Adrenal metastases are also typically asymptomatic and are usually discovered by routine CT scan. They may lead to adrenal hypofunction. Skin and soft tissue metastases occur in 8% of patients dying of lung cancer and generally present as painless subcutaneous or intramuscular masses. Occasionally, the tumor erodes through the overlying skin, with necrosis and creation of a chronic wound; excision may then be necessary for both mental and physical palliation.
Lung cancer often produces a variety of nonspecific symptoms such as anorexia, weight loss, fatigue, and malaise. The cause of these symptoms is often unclear, but should raise concern about possible metastatic disease.
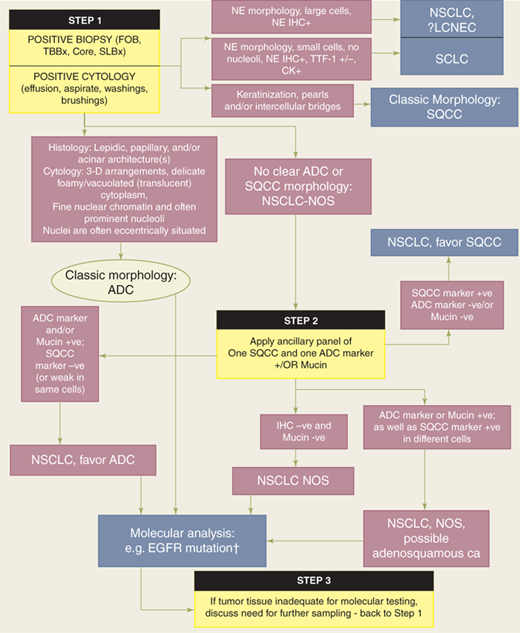
Establishing a clear histologic diagnosis early in the evaluation and management of lung cancer is critical to effective treatment. Molecular signatures are also key determinants of treatment algorithms for adenocarcinoma and will likely become important for squamous cell carcinoma as well. Currently, differentiation between adenocarcinoma and squamous cell carcinoma in cytologic specimens or small biopsy specimens is imperative in patients with advanced stage disease, as treatment with pemetrexed or bevacizumab-based chemotherapy is associated with improved progression-free survival in patients with adenocarcinoma but not squamous cell cancer. Furthermore, life-threatening hemorrhage has occurred in patients with squamous cell carcinoma who were treated with bevacizumab. Finally, EGFR mutation predicts response to EGFR tumor kinase inhibitors and is now recommended as first-line therapy in advanced adenocarcinoma. Because adequate tissue is required for histologic assessment and molecular testing, each institution should have a clear, multidisciplinary approach to patient evaluation, tissue acquisition, tissue handling/processing, and tissue analysis (Fig. 19-18). In many cases, tumor morphology differentiates adenocarcinoma from the other histologic subtypes. If no clear morphology can be identified, then additional testing for one immunohistochemistry marker for adenocarcinoma and one for squamous cell carcinoma will usually enable differentiation. Immunohistochemistry for neuroendocrine markers is reserved for lesions exhibiting neuroendocrine morphology. Additional molecular testing should be performed on all adenocarcinoma specimens for known predictive and prognostic tumor markers (e.g. EGFR, KRAS, and EML4-ALK fusion gene). Ideally, use of tissue sections and cell block material is limited to the minimum necessary at each decision point. This emphasizes the importance of a multidisciplinary approach; surgeons and radiologists must work in direct cooperation with the cytopathologist to ensure that tissue samples are adequate for morphologic diagnosis as well as providing sufficient cellular material to enable molecular testing. With adoption of endobronchial and endoscopic ultrasound, electromagnetic navigational bronchoscopy, VATS, and even transthoracic image-guided fine-needle and core-needle biopsy, surgeons are increasingly involved in the acquisition of diagnostic tissue for primary, metastatic, and recurrent intrathoracic disease, and a thorough understanding of key issues is necessary to ensure optimal treatment and patient outcomes.
Figure 19-18.
Algorithm for adenocarcinoma diagnosis in small biopsies and/or cytology. Step 1: When positive biopsies (fiberoptic bronchoscopy [FOB], transbronchial [TBBx], core, or surgical lung biopsy [SLBx]) or cytology (effusion, aspirate, washings, and brushings) show clear adenocarcinoma (ADC) or squamous cell carcinoma (SQCC) morphology, the diagnosis can be firmly established. If there is neuroendocrine (NE) morphology, the tumor may be classified as small cell carcinoma (SCLC) or non–small cell lung carcinoma (NSCLC), probably large cell neuroendocrine carcinoma (LCNEC) according to standard criteria (+ = positive, – = negative, and ± = positive or negative). If there is no clear ADC or SQCC morphology, the tumor is regarded as NSCLC–not otherwise specified (NOS). Step 2: NSCLC-NOS can be further classified based on (a) immunohistochemical stains, (b) mucin (DPAS or mucicarmine) stains, or (c) molecular data. If the stains all favor ADC-positive ADC marker(s) (i.e., TTF-1 and/or mucin positive) with negative SQCC markers, then the tumor is classified as NSCLC, favor ADC. If SQCC markers (i.e., p63 and/or CK5/6) are positive with negative ADC markers, the tumor is classified as NSCLC, favor SQCC. If the ADC and SQCC markers are both strongly positive in different populations of tumor cells, the tumor is classified as NSCLC-NOS, with a comment it may represent adenosquamous carcinoma. If all markers are negative, the tumor is classified as NSCLC-NOS. †EGFR mutation testing should be performed in (1) classic ADC, (2) NSCLC, favor ADC, (3) NSCLC-NOS, and (4) NSCLC-NOS, possible adenosquamous carcinoma. In NSCLC-NOS, if EGFR mutation is positive, the tumor is more likely to be ADC than SQCC. Step 3: If clinical management requires a more specific diagnosis than NSCLC-NOS, additional biopsies may be indicated. CD = cluster designation; CK = cytokeratin; DPAS = diastase-periodic acid Schiff; DPAS +ve = periodic-acid Schiff with diastase; EGFR = epidermal growth factor receptor; IHC = immunohistochemistry; NB = of note; TTF-1 = thyroid transcription factor-1; –ve = negative; +ve = positive. (Reproduced with permission from Travis W, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244.
Evaluation prior to treatment encompasses three areas: diagnosis and assessment of the primary tumor, assessment for metastatic disease, and determination of functional status (the patient’s ability to tolerate the prescribed treatment regimen). A discrete approach to each area allows the surgeon to systematically evaluate the patient, perform accurate clinical staging, and enable assessment of the patient’s functional suitability for therapy, including pulmonary resection (Table 19-9).
| PRIMARY TUMOR | METASTATIC DISEASE | FUNCTIONAL ASSESSMENT | |
|---|---|---|---|
| History | Pulmonary | Weight loss | Ability to walk up two flights of stairs |
| Nonpulmonary thoracic | Malaise | Ability to walk on a flat surface indefinitely | |
| Paraneoplastic | New bone pain | ||
| Neurologic signs or symptoms | |||
| Skin lesions | |||
| Physical examination | Voice | Supraclavicular node palpation | Accessory muscle usage |
| Skin examination | Air flow by auscultation | ||
| Neurologic examination | Force of cough | ||
| Radiographic examination | Chest CT | Chest CT, PET | Chest CT: tumor anatomy, atelectasis |
| Tissue analysis | Bronchoscopy | Bone scan, head MRI, abdominal CT | Quantitative perfusion scan |
| Transthoracic needle aspiration and biopsy | Bronchoscopic lymph node FNA | ||
| Endoscopic ultrasound | |||
| Mediastinoscopy | |||
| Biopsy of suspected metastasis | |||
| Other | Thoracoscopy | — | Pulmonary function tests (FEV1, Dlco, O2 consumption) |
Assessment of the primary tumor begins with the history and directed questions regarding the presence or absence of pulmonary, nonpulmonary, thoracic, and paraneoplastic symptoms. Because patients often present to the surgeon with a CXR or CT scan demonstrating the lesion, the location of the tumor can help direct the clinician in performing the history and physical examination.
If the patient does not already have a chest CT scan, this should be performed expeditiously as the next stage in evaluating a new patient. A routine chest CT scan should include intravenous contrast material to enable assessment of the primary tumor, delineation of mediastinal lymph nodes relative to normal mediastinal structures, and the tumor’s relationship to surrounding and contiguous structures. Recommendations for treatment and options for obtaining tissue diagnosis require a thorough understanding and assessment of CT findings.
Concern for contiguous invasion of adjacent structures often is raised in response to a combination of symptom history, location of the primary tumor, and CT imaging. It is common to see the primary tumor abutting the chest wall without clear radiographic evidence of rib destruction. In this circumstance, a history of pain in the area is an accurate guide to the likelihood of parietal pleural, rib, or intercostal nerve involvement. Similar observations apply to tumors abutting the recurrent laryngeal nerve, phrenic nerve, diaphragm, vertebral bodies, and chest apex. Thoracotomy should not be denied because of presumptive evidence of invasion of the chest wall, vertebral body, or mediastinal structures; proof of invasion may require thoracoscopy or even thoracotomy.
MRI of pulmonary lesions and mediastinal nodes, overall, offers no real improvement over CT scanning. It is an excellent modality, however, for defining a tumor’s relationship to a major vessel, given its excellent imaging of vascular structures. This is especially true if the use of iodine contrast material is contraindicated. Thus, use of MRI in lung cancer patients is reserved for those with contrast allergies or with suspected mediastinal, vascular, or vertebral body invasion.
The surgeon must have an evidence-based algorithm for approaching the diagnosis and treatment of a pulmonary nodule and masses (Fig. 19-19).24 Depending on nodule size, proximity to the bronchial tree, and the prevalence of cancer in the population being sampled, bronchoscopy has 20% to 80% sensitivity for detecting neoplastic processes within a pulmonary lesion. Diagnostic tissue from bronchoscopy can be obtained by one of four methods:
Figure 19-19.
Recommended management algorithm for patients with solitary pulmonary nodules (SPNs) measuring 8 mm to 30 mm in diameter. CT = computed tomography; CXR = chest radiograph; PET = positron emission tomography; XRT = radiotherapy. (Reproduced with permission from the American College of Chest Physicians from Gould MK, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP Evidence-based Clinical Practice Guidelines. (2nd edition) Chest. 2007;132:108S.)
Brushings and washings for cytology
Direct forceps biopsy of a visualized lesion
Endobronchial ultrasound-guided fine-needle aspiration (FNA) of an externally compressing lesion without visualized endobronchial tumor
Transbronchial biopsy with fluoroscopy to guide forceps to the lesion or electromagnetic navigational bronchoscopy
Electromagnetic navigation bronchoscopy is a recent addition to the surgeon’s armamentarium for transbronchial biopsy of peripheral lung lesions. Using electromagnetic markers that create a three-dimensional image and align the recorded CT images to the patient’s true anatomy, a catheter is advanced transbronchially and brushings, FNA, cup biopsy, and washings can be performed. Diagnostic yield using electromagnetic navigation bronchoscopy as an adjunct to standard bronchoscopy is reported as high as 80%. The approach can also be used for placement of fiducial markers for subsequent stereotactic body radiation therapy and for tattooing the perilesional region to guide subsequent video-assisted thoracoscopic resection. Pneumothorax rates with this approach are approximately 1% in larger series and up to 3.5% in reports of early experience.
For peripheral lesions (roughly the outer half of the lung), transbronchial biopsy is performed first, followed by brushings and washings. This improves diagnostic yield by disrupting the lesion with the biopsy forceps and mobilizing additional cells. For central lesions, direct forceps biopsy by bronchoscopic visualization is often possible. For central lesions with external airway compression but no visible endobronchial lesions, endobronchial ultrasound (EBUS) is highly accurate and safe for transbronchial biopsies of both the primary tumor (when it abuts the central airways) as well as the mediastinal lymph nodes.33
Image-guided transthoracic FNA (ultrasound or CT FNA) biopsy can accurately diagnose appropriately selected peripheral pulmonary lesions in up to 95% of patients. Three biopsy results are possible after image-guided biopsy procedures: malignant, a specific benign process, or indeterminate. Because false-negative rates range from 3% to 29%, further diagnostic efforts are warranted in the absence of a specific benign diagnosis (such as granulomatous inflammation or hamartoma) because malignancy is not ruled out.34 The primary complication is pneumothorax in as many as 30% of cases. Intrapulmonary bleeding occurs, but rarely causes clinically significant hemoptysis or respiratory compromise.
Some groups advocate use of video-assisted thoracoscopic biopsy as the first option for diagnosis, citing superior diagnostic accuracy and low surgical risk. With VATS, the nodule can be excised with a wedge or segmental resection, if less than 3 cm, or a core-needle biopsy can be performed under direct vision for larger lesions. VATS can also provide valuable staging information, including sampling/dissection of mediastinal lymph nodes and assessing whether the primary tumor has invaded a contiguous structure (such as the chest wall or mediastinum). Lesions most suitable for VATS are those that are located in the outer one third of the lung. The surgeon should avoid direct manipulation of the nodule or violation of the visceral pleura overlying the nodule. In addition, the excised nodule must be extracted from the chest within a bag to prevent seeding of the chest wall. If the patient’s pulmonary reserve is adequate, the surgeon can proceed to lobectomy (either VATS or open) after frozen section diagnosis.
A thoracotomy is occasionally necessary to diagnose and stage a primary tumor. Although this occurs rarely, two circumstances may require such an approach: (a) a deep-seated lesion that yielded an indeterminate needle biopsy result or that could not be biopsied for technical reasons; or (b) inability to determine invasion of a mediastinal structure by any method short of palpation. In the circumstance of a deep-seated lesion without a diagnosis, tissue can be obtained via thoracotomy using FNA, core-needle biopsy, or excisional biopsy. Intraoperative frozen-section analysis is required; if the open biopsy frozen-section result is indeterminate, a lobectomy may be necessary in extremely rare situations. If a pneumonectomy is required to remove the lesion, a tissue diagnosis of cancer must be made before proceeding.
Distant metastases are found in approximately 40% of patients with newly diagnosed lung cancer. The presence of lymph node or systemic metastases may imply inoperability. As with the primary tumor, assessment for the presence of metastatic disease should begin with the history and physical examination, focusing on the presence or absence of new bone pain, neurologic symptoms, and new skin lesions. In addition, constitutional symptoms (e.g., anorexia, malaise, and unintentional weight loss of >5% of body weight) suggest either a large tumor burden or the presence of metastases. Physical examination should focus on the patient’s overall appearance, noting any evidence of weight loss such as redundant skin or muscle wasting, and a complete examination of the head and neck, including evaluation of cervical and supraclavicular lymph nodes and the oropharynx. This is particularly true for patients with a significant tobacco history. The skin should be thoroughly examined. Routine laboratory studies include serum levels of hepatic enzymes (e.g., serum glutamic oxaloacetic transaminase and alkaline phosphatase), as well as those of serum calcium (to detect bone metastases or the ectopic parathyroid syndrome). Elevation of either hepatic enzymes or serum calcium levels typically occurs with extensive metastases.
Chest CT scanning permits assessment of possible metastatic spread to the mediastinal lymph nodes. It continues to be the most effective noninvasive method available to assess the mediastinal and hilar nodes for enlargement. However, a positive CT result (i.e., nodal diameter >1.0 cm) predicts actual metastatic involvement in only about 70% of lung cancer patients. Thus even with enlarged mediastinal lymph nodes on a CT scan, up to 30% of such nodes are enlarged from noncancerous reactive causes such as inflammation due to atelectasis or pneumonia secondary to the tumor. Therefore, no patient should be denied an attempt at curative resection just because of a positive CT result for mediastinal lymph node enlargement. Any CT finding of metastatic nodal involvement must be confirmed histologically. The negative predictive value of normal-appearing lymph nodes by CT (lymph nodes <1.0 cm) is better than the positive predictive value of a suspicious-appearing lymph node, particularly with small squamous cell tumors. With normal-size lymph nodes and a T1 tumor, the false-negative rate is less than 10%, leading many surgeons to omit mediastinoscopy. However, the false-negative rate increases to nearly 30% with centrally located and T3 tumors. It has also been demonstrated that T1 adenocarcinomas or large cell carcinomas have a higher rate of early micrometastasis. Therefore, all such patients should undergo mediastinoscopy.
Mediastinal lymph node staging by PET scanning appears to have greater accuracy than CT scanning. PET staging of mediastinal lymph nodes has been evaluated in two meta-analyses. The overall sensitivity for mediastinal lymph node metastasis was 79% (95% confidence interval 76%–82%), with a specificity of 91% (95% CI 89%–93%) and an accuracy of 92% (95% CI 90%–94%).35
In comparing PET with CT scans in patients who also underwent lymph node biopsies, PET had a sensitivity of 88% and a specificity of 91%, whereas CT scanning had a sensitivity of 63% and a specificity of 76%. Combining CT and PET scanning may lead to even greater accuracy.36 In one study of CT, PET, and mediastinoscopy in 68 patients with potentially operable NSCLC, CT correctly identified the nodal stage in 40 patients (59%). It understaged the tumor in 12 patients and overstaged it in 16 patients. PET correctly identified the nodal stage in 59 patients (87%). It understaged the tumor in five patients and overstaged it in four. For detecting N2 and N3 disease, the combination of PET and CT scanning yielded a sensitivity, specificity, and accuracy of 93%, 95%, and 94%, respectively. CT scan alone yielded 75%, 63%, and 68%, respectively. Studies examining combined PET-CT consistently show improved accuracy compared to PET or CT alone; accuracy for PET-CT nodal positivity confirmed by mediastinoscopy is approximately 75%, with a negative predictive value of approximately 90%. Right upper lobe lesions were more likely to have occult N2 disease than other lobes of the lung.37,38,39,40 PET-positive mediastinal lymph nodes require histologic verification of node positivity, either by EBUS-guided FNA/core-needle biopsy or mediastinoscopy, to minimize the risk of undertreatment. Assuming node positivity without histologic confirmation relegates the patient to, at a minimum, induction chemotherapy. If there is a suggestion of N3 disease, the patient would be incorrectly staged as having IIIB disease and would not be considered a candidate for potentially curative surgical resection.
It is important for surgeons who are managing patients with lung cancer to have a clear algorithm for invasive mediastinal staging. In general, invasive staging is underused, placing many patients at risk for over- or understaging and, thus, inappropriate treatment. An absolute indication for obtaining a tissue diagnosis is mediastinal lymph node enlargement greater than 1.0 cm by CT scan. There are several options for invasive mediastinal staging:
Less invasive than mediastinoscopy, EBUS enables image-guided transtracheal and transbronchial FNA cytologic samples from hilar masses and lymph nodes from level 4R and 4L, level 7, level 10, and level 11. A core-needle biopsy device recently became available for the EBUS and will improve the diagnostic yield when sampling mediastinal lymphadenopathy. Rapid onsite pathologic evaluation with expert cytopathologist evaluation greatly increases the diagnostic accuracy of the procedure; importantly, the intraoperative evaluation will confirm whether the target lesion is being sampled and greatly facilitates acquisition of satisfactory samples for determining the morphologic diagnosis as well as sufficient material for cell block for immunohistochemistry and molecular testing. Like mediastinoscopy, EBUS does not allow assessment of level 3, 5, or 6 nodal stations.
Endoscopic ultrasound (EUS) can accurately visualize mediastinal paratracheal lymph nodes (stations 4R, 7, and 4L) and other lymph node stations (stations 8 and 9) and is able to visualize primary lung lesions contiguous with or near the esophagus (see Fig. 19-8). Using FNA or core-needle biopsy, samples of lymph nodes or primary lesions can be obtained. Diagnostic yield is improved with intraoperative cytologic evaluation, which can be performed with the cytopathologist in the operating room. Limitations of EUS include the inability to visualize the anterior (pretracheal) mediastinum; thus, EUS does not replace mediastinoscopy for complete mediastinal nodal staging. However, it may not be necessary to perform mediastinoscopy if findings on EUS are positive for N2 nodal disease, particularly if more than one station is found to harbor metastases.
Cervical mediastinoscopy provides tissue sampling of all paratracheal and subcarinal lymph nodes and permits visual determination of the presence of extracapsular extension of nodal metastasis (Fig. 19-20). With complex hilar or right paratracheal primary tumors, it allows direct biopsies and assessment of invasion into the mediastinum. When the size of mediastinal lymph nodes is normal, mediastinoscopy is generally recommended for centrally located tumors, for T2 and T3 primary tumors, and occasionally for T1 adenocarcinomas or large cell carcinomas (due to their higher rate of metastatic spread). Some surgeons perform mediastinoscopy in all lung cancer patients because of the poor survival associated with surgical resection of N2 disease.
It is important to note that EBUS or EUS can be used for initial diagnosis in enlarged lymph nodes, but the predictive value of a negative EBUS in a patient with radiographically suspicious mediastinal disease is not sufficient to accurately guide treatment. At the authors’ institutions, it is standard to begin mediastinal lymph node staging with EBUS-guided FNA of clinically suspicious mediastinal lymphadenopathy. If the FNA is negative by intraoperative rapid onsite cytologic evaluation, cervical video mediastinoscopy is performed in the same operative setting to ensure accurate staging of the mediastinal nodes. However, if the FNA is positive, mediastinoscopy is not performed and the patient is referred to medical oncology for induction chemotherapy; avoiding a pretreatment mediastinoscopy in this manner facilitates the safe performance of a postinduction mediastinoscopy for restaging of the mediastinum in patients who respond favorably to induction therapy.
Left video-assisted thoracoscopic lymph node sampling may be needed for patients with left upper lobe tumors who have localized regional spread to station 5 and 6 lymph nodes, without mediastinal paratracheal involvement (see Fig. 19-8). If there is a low index of suspicion for nodal metastasis, the patient can be schedule for VATS biopsy and lobectomy under the same anesthesia; the procedure begins by sampling the level 5 and 6 nodes for frozen section, and if the nodes are negative, the anatomic lung resection is performed. If the index of suspicion is high, the VATS biopsy is performed as a separate procedure. Cervical mediastinoscopy should precede VATS biopsy, even if patients have normal paratracheal lymph nodes. Additional diagnostic evaluation of the lymph nodes in station 5 and 6 may be unnecessary if the mediastinal lymph nodes are proven to be benign with biopsy during cervical mediastinoscopy and the preoperative CT scan suggests complete respectability of the tumor. There are, however, several indications for prethoracotomy biopsy of station 5 and 6 lymph nodes, which are listed in Table 19-10. It is particularly important to prove that mediastinal lymph nodes are pathologically involved and not just radiographically suspicious for nodal metastasis prior to deciding that the patient is not a candidate for resection.
1. Enrollment criteria for induction therapy protocol require pathologic confirmation of N2 disease. 2. Computed tomographic scan shows evidence of bulky nodal metastases or extracapsular spread that could prevent complete resection. 3. Tissue diagnosis of a hilar mass or of lymph nodes causing recurrent laryngeal nerve paralysis is needed. |
The presence of pleural effusion on radiographic imaging should not be assumed to be malignant. Pleural effusion may be secondary to atelectasis or consolidation, seen with central tumors, reactive, or secondary to cardiac dysfunction. Pleural effusion associated with a peripherally based tumor, particularly one that abuts the visceral or parietal pleural surface, does have a higher probability of being malignant, which would alter the pathologic stage of the disease to stage IV by the American Joint Committee on Cancer (AJCC) 7th edition TNM staging criteria. If this is the only site concerning for metastatic disease, pathologic confirmation is mandatory. Cytology reveals malignant cells in 50% of malignant effusions. Thoracoscopy, performed as part of a separate staging procedure, often with mediastinoscopy or immediately before a planned thoracotomy, may be needed to rule out pleural metastases in select patients.
Currently, chest CT and PET are routine in the evaluation of patients with lung cancer. Integrated PET-CT scanners have become standard and have substantially improved accuracy of detection and localization of lymph node and distant metastases, as compared with independently performed PET and CT scans (Fig. 19-21). This technology overcomes the imprecise information on the exact location of focal abnormalities seen on PET and has become the standard imaging modality for lung cancer. Compared to routine chest or abdominal CT and bone scans, PET scanning detects 10% to 15% more distant metastases, but should be confirmed with MRI and/or biopsies if the patient otherwise has early-stage disease. Brain MRI should be performed when the suspicion or risk of brain metastases is increased, such as in patients with clinical stage III disease. In the absence of neurologic symptoms or signs, the probability of a negative head CT scan is 95%. Liver abnormalities that are not clearly simple cysts or hemangiomas and adrenal enlargement, nodules, or masses are further evaluated by MRI scanning and, occasionally, by needle biopsy. Adrenal adenomas have a high lipid content (secondary to steroid production), but metastases and most primary adrenal malignancies contain little if any lipid; thus MRI is usually able to distinguish the two.
Figure 19-21.
Imaging of non–small cell lung cancer by integrated positron emission tomography (PET)-computed tomography (CT) scan. A. CT of the chest showing a tumor in the left upper lobe. B. PET scan of the chest at the identical cross-sectional level. C. Coregistered PET-CT scan clearly showing tumor invasion (confirmed intraoperatively). (Reprinted with permission from Lardinois D, Weder W, Hany TF, et al. Staging of non-small–cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2504. Copyright © 2003 Massachusetts Medical Society.)
The staging of any tumor is an attempt to estimate the extent of disease and determine the patient’s prognosis; in a given patient, tumors are typically classified into a clinical stage and a pathologic stage. Clinical staging information includes the history and physical examination, radiographic test results, and diagnostic biopsy information. Therapeutic plans are generated based on clinical stage. After surgical resection of the tumor and lymph nodes, a postoperative pathologic stage (pTNM) is determined, providing further prognostic information.
The staging of solid epithelial tumors is based on the TNM staging system. The primary tumor “T” status provides information about tumor size and relationship to surrounding structures; the “N” status provides information about regional lymph nodes; and the “M” status provides information about the presence or absence of metastatic disease. The designation of lymph nodes as N1, N2, or N3 requires familiarity with the lymph node mapping system41 (see Fig. 19-8). Based on clearly delineated anatomic boundaries, accurate and reproducible localization of thoracic lymph nodes is possible, which facilitates detailed nodal staging for individual patients and standardization of nodal assessment between surgeons.
Pathologic staging criteria are based on the predicted survival relative to each combination of tumor, node, and metastasis status. In 2010, the AJCC 7th edition incorporated multiple changes into the staging system for NSCLC based on analysis of survival predictors from more than 100,000 patients worldwide. Table 19-11 shows the clinical and pathologic criteria for each of the TNM descriptors currently used in staging NSCLC and the overall stage classifications. In addition to the TNM stage, it is recommended that histologic grade, presence or absence of pleural/elastic layer invasion, separate tumor nodules, lymphovascular invasion, and residual tumor after treatment also be recorded into cancer registries to facilitate evaluation of these potential predictors in future analysis of staging criteria.
| CLINICAL Extent of disease before any treatment | Stage Category Definitions | PATHOLOGIC Extent of disease through completion of definitive surgery | |
|---|---|---|---|
| ❏ y clinical–staging completed after neoadjuvant therapy but before subsequent surgery | TUMOR SIZE: ———— | LATERALITY:❏ left ❏ right ❏ bilateral | ❏ y pathologic–staging completed after neoadjuvant therapy AND subsequent surgery |
❏ TX ❏ TO ❏ Tis ❏ T1 ❏ T1a ❏ T1b ❏ T2 ❏ T2a ❏ T2b ❏ T3 ❏ T4 | Primary Tumor (T) Primary tumor cannot be assessed No evidence of primary tumor Tis Carcinoma in situ Tumor ≤3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus)* Tumor ≤2 cm in greatest dimension Tumor > 2 cm but ≤3 cm in greatest dimension Tumor > 3 cm but ≤7 cm or tumor with any of the following features (T2 tumors with these features are classified T2a if ≤5 cm) Involves main bronchus, ≥2 cm distal to the carina Invades visceral pleura (PL1 or PL2) Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung Tumor > 3 cm but ≤5 cm in greatest dimension Tumor > 5 cm but ≤7 cm in greatest dimension Tumor > 7 cm or one that directly invades any of the following: parietal pleural (PL3) chest wall (including superior sulcus tumors), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium; or tumor in the main bronchus (< 2 cm distal to the carina* but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumor nodule(s) in the same lobe Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina, separate tumor nodule(s) in a different ipsilateral lobe * The uncommon superficial spreading tumor of any size with its invasive component limited to the bronchial wall, which may extend proximally to the main bronchus, is also classified as T1a. | ❏ TX ❏ TO ❏ Tis ❏ T1 ❏ T1a ❏ T1b ❏ T2 ❏ T2a ❏ T2b ❏ T3 ❏ T4 | |
❏ NX ❏ NO ❏ N1 ❏ N2 ❏ N3 | Regional Lymph Nodes (N) Regional lymph nodes cannot be assessed No regional lymph node metastasis Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension Metastasis in ipsilateral mediastinal and/or subcarinal llymph node(s) Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) | ❏ NX ❏ NO ❏ N1 ❏ N2 ❏ N3 | |
❏ MO ❏ M1 ❏ M1a ❏ M1b | Distant Metastasis (M) No distant metastasis (no pathologic MO; use clinical M to complete stage group) Distant metastasis Separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural (or pericardial) effusion** Distant metastasis **Most pleural (and pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple cytopathologic examinations of pleural (pencardial) fluid are negative for tumor, and the fluid is nonbloody and is not an exudate. Where these elements and clinical judgement dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element and the patient should be classified as MO. | ❏ M1 ❏ M1a ❏ M1b | |
Staging for small cell lung cancer (SCLC) is typically based on the extent of disease. SCLC presenting with bulky locoregional disease confined to the ipsilateral hemithorax, with no evidence for distant metastatic disease, is termed “limited” SCLC. Limited disease must be treatable within a tolerable field of radiation. Using AJCC descriptors, this includes any T stage, any N stage, without metastatic disease (M0). The only exception is when multiple lung nodules are widely spread throughout the ipsilateral lung in the same hemithorax; in these patients, the size of the involved area would preclude a “safe” radiation field. In contrast, in “disseminated” disease, tumor is beyond the ipsilateral hemithorax or widely spread within the ipsilateral lung and to distant sites. Metastases to the pleura and pericardium, with resultant effusions, are considered disseminated disease. Metastases to brain, bone, bone marrow, and the pleural and pericardial spaces are common.
Patients with potentially resectable tumors require careful assessment of their functional status and ability to tolerate either lobectomy or pneumonectomy. The surgeon should first estimate the likelihood of pneumonectomy, lobectomy, or possibly sleeve resection, based on the CT images. A sequential process of evaluation then unfolds.
A patient’s history is the most important tool for gauging risk. Specific questions regarding performance status should be routinely asked. If the patient can walk on a flat surface indefinitely, without oxygen and without having to stop and rest secondary to dyspnea, he will be very likely to tolerate lobectomy. If the patient can walk up two flights of stairs (up two standard levels), without having to stop and rest secondary to dyspnea, she will likely tolerate pneumonectomy. Finally, nearly all patients, except those with carbon dioxide (CO2) retention on arterial blood gas analysis, will be able to tolerate periods of single-lung ventilation and wedge resection.
Current smoking status and sputum production are also pertinent. Current smokers and patients with a greater than 60 pack-year history of smoking have a significantly increased risk of postoperative pulmonary complications; heavy smokers are 2.5 times more likely to develop pulmonary complications and three times more likely to develop pneumonia compared to patients with a ≤60 pack-year history (odds ratio [OR] 2.54; 95% CI 1.28–5.04; P = .0008). Impaired exchange of CO2 is also predictive of increased risk, independent of the smoking history. For every 10% decline in percent carbon monoxide diffusion capacity (%Dlco), the risk of any pulmonary complication increased by 42% (OR 1.42; 95% CI 1.16–1.75; P = .008).42 Risk reduction requires smoking cessation at least 8 weeks preoperatively, a requirement that is often not feasible in a cancer patient. Nevertheless, abstinence for at least 2 weeks before surgery should be encouraged. Smoking cessation on the day of surgery leads to increased sputum production and potential secretion retention postoperatively, and some authors have reported increased rates of pulmonary complications in this group.43 Patients with chronic daily sputum production will have more problems postoperatively with retention and atelectasis; they are also at higher risk for pneumonia. Sputum culture, antibiotic administration, and bronchodilators may be warranted preoperatively.
Pulmonary function studies are routinely performed when any resection greater than a wedge resection will be performed. Of all the measurements available, the two most valuable are forced expiratory volume in 1 second (FEV1) and carbon monoxide diffusion capacity (Dlco). General guidelines for the use of FEV1 in assessing the patient’s ability to tolerate pulmonary resection are as follows: greater than 2.0 L can tolerate pneumonectomy, and greater than 1.5 L can tolerate lobectomy. It must be emphasized that these are guidelines only. It is also important to note that the raw value is often imprecise because normal values are reported as “percent predicted” based on corrections made for age, height, and gender. For example, a raw FEV1 value of 1.3 L in a 62-year-old, 75-inch male has a percent predicted value of 30% (because the normal expected value is 4.31 L); in a 62-year-old, 62-inch female, the predicted value is 59% (normal expected value 2.21 L). The male patient is at high risk for lobectomy, while the female could potentially tolerate pneumonectomy.
To calculate the predicted postoperative value for FEV1 or Dlco, the percent predicted value of FEV1 or Dlco is multiplied by the fraction of remaining lung after the proposed surgery. For example, with a planned right upper lobectomy, a total of three segments will be removed. Therefore, three of a total 20 segments will leave the patient with (20 – 3/20) × 100 = 85% of their original lung capacity. In the two patients mentioned earlier, the man will have a predicted postoperative FEV1 of 30% × 0.85 = 25%, whereas the woman will have a predicted postoperative FEV1 of 50%. Percent predicted value of less than 50% for either FEV1 or Dlco correlates with risk for postoperative complications, particularly pulmonary complications; the risk of complications increases in a stepwise fashion for each 10% decline. Figure 19-22 shows the relationship between predicted postoperative Dlco and estimated operative mortality.
Figure 19-22.
Operative mortality after major pulmonary resection for non–small cell lung cancer (334 patients) as a function of percent predicted postoperative carbon monoxide diffusion capacity (ppoDlco%). Solid line indicates logistic regression model; dashed lines indicate 95% confidence limits. (Adapted with permission from Wang J, Olak K, Ferguson M. Diffusing capacity predicts operative mortality but not long-term survival after resection for lung cancer. J Thorac Cardiovasc Surg. 1999;117:582. Copyright Elsevier.)
Quantitative perfusion scanning is used in select circumstances to help estimate the functional contribution of a lobe or whole lung. Such perfusion scanning is most useful when the impact of a tumor on pulmonary physiology is difficult to discern. With complete collapse of a lobe or whole lung, the impact is apparent, and perfusion scanning is usually unnecessary. Figure 19-23 shows a tumor with significant right main stem airway obstruction with associated atelectasis and volume loss of the right lung. At presentation, the patient was dyspneic with ambulation, and the FEV1 was 1.38 L. Six months prior, this patient could walk up two flights of stairs without dyspnea. The surgeon can anticipate that the patient will tolerate pneumonectomy because the lung is already not functioning due to main stem airway obstruction, and may, in fact, be contributing to a shunt. However, with centrally located tumors associated with partial obstruction of a lobar or main bronchus or of the pulmonary artery, perfusion scanning may be valuable in predicting the postoperative result of resection. For example, if the quantitative perfusion to the right lung is measured to be 21% (normal is 55%) and the patient’s percent predicted FEV1 is 60%, the predicted postoperative FEV1 after a right pneumonectomy would be 60% × 0.79 = 47%, indicating the ability to tolerate pneumonectomy. If the perfusion value is 55%, the predicted postoperative value would be 27%, and pneumonectomy would pose a significantly higher risk.
It is not uncommon to encounter patients with significant reductions in their percent predicted FEV1 and Dlco whose history shows a functional status that is inconsistent with the pulmonary function tests. In these circumstances, exercise testing that yields maximal oxygen consumption (V.o2 max) has emerged as a valuable decision-making technique to help patients with abnormal FEV1 and Dlco (Table 19-12). Values of less than 10 mL/kg/min are associated with a 26% mortality after major pulmonary resection compared to only 8.3% with V.o2 max ≥10 mL/kg/min. Values greater than 15 mL/kg/min generally indicate the patient’s ability to tolerate pneumonectomy.
| STUDY | DEATHS/TOTAL |
|---|---|
| V.o2 max 10–15 mL/kg per minute | |
| Smith et al196 | 1/6 (33%) |
| Bechard and Wetstein197 | 0/15 (0%) |
| Olsen et al198 | 1/14 (7.1%) |
| Walsh et al199 | 1/5 (20%) |
| Bolliger et al200 | 2/17 (11.7%) |
| Markos et al201 | 1/11 (9.1%) |
| Wang et al202 | 0/12 (0%) |
| Win et al203 | 2/16 (12.5%) |
| Total | 8/96 (8.3%) |
| V.o2 max <10 mL/kg per minute | |
| Bechard and Wetstein197 | 2/7 (29%) |
| Olsen et al198 | 3/11 (27%) |
| Holden et al204 | 2/4 (50%) |
| Markos et al201 | 0/5 (0%) |
| Total | 7/27 (26%) |
The risk assessment of a patient is an amalgam of clinical judgment and data. The risk assessment described earlier must be integrated with the experienced clinician’s sense of the patient and with the patient’s attitude toward the disease and toward life. Figure 19-24 provides a useful algorithm for determining suitability for lung resection.44
Figure 19-24.
Algorithm for preoperative evaluation of pulmonary function and reserve prior to resectional lung surgery. CPET = cardiopulmonary exercise test; CT = computed tomographic scan; CXR = chest radiograph; Dlco = carbon monoxide diffusion capacity; FEV1 = forced expiratory volume in 1 second; %ppo = percent predicted postoperative lung function; V.o2 max = maximum oxygen consumption. (Reproduced with permission from the American College of Chest Physicians from Colice GL, et al: Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP Evidence-based Clinical Practice Guidelines. (2nd edition) Chest. 2007;132:161S.)
In rare circumstances where SCLC presents as an isolated lung lesion, lobectomy followed by chemotherapy is warranted after surgical mediastinal staging has confirmed the absence of N2 disease. Often, ultrasound-guided FNA provides a definitive positive diagnosis and more invasive approaches are not needed. However, less than 5% are stage I, and there is no benefit from surgical resection for more advanced-stage disease; treatment is chemotherapy with or without radiation therapy depending on the extent of disease and the patient performance status.
Early-stage disease includes T1 and T2 tumors (with or without N1 nodal involvement) and T3 tumors (without N1 nodal involvement). This group represents a small but increasing proportion of the total number of patients diagnosed with lung cancer each year (approximately 20% of 101,844 patients from 1989 to 2003).45 Surgical resection is the current standard, ideally accomplished by video-assisted lobectomy or pneumonectomy, depending on the tumor location.
Despite the term “early-stage,” the overall 5-year survival rate for stage I is 65% and only 41% for stage II. Median survival for untreated patients with stage IA NSCLC is 14 months, and 5-year survival rate is 22%.46 After surgical resection of postoperative pathologic stage IA disease, 5-year survival is better than with no treatment, but still only 67%.41 Survival declines with higher stages. Advanced age at diagnosis, male sex, low socioeconomic status, nonsurgical treatment, and poor histologic grade are associated with increased mortality risk on multivariate analysis.45
Depending on tumor size and location, lobectomy, sleeve lobectomy, and occasionally pneumonectomy, with mediastinal lymph node dissection or sampling, are appropriate for patients with clinical early-stage disease. Sleeve resection is performed for tumors located at airway bifurcations when an adequate bronchial margin cannot be obtained by standard lobectomy. Pneumonectomy is rarely performed; primary indications for pneumonectomy in early-stage disease include large central tumors involving the distal main stem bronchus and inability to completely resect involved N1 lymph nodes. The latter circumstance occurs with bulky adenopathy or with extracapsular nodal spread.
Lobectomy may not be an option for some patients with early-stage disease, due to poor cardiopulmonary function or other comorbid illnesses. The ultimate decision that a patient is not operable, both with regard to the ability of the patient to tolerate surgery and the likelihood of successful resection, should be accepted only after evaluation by an expert surgeon. Surgeons with limited expertise, when faced with a complicated patient, should refer the patient to a high-volume center for further evaluation if they are unable to offer the patient surgical resection in their own center.
Limited resection, defined as segmentectomy or wedge resection, is a viable option for achieving local control in high-risk patients. Historically, limited resection with wedge or segmentectomy has been considered a compromise operation due to unacceptably high rates of local recurrence and concerns for worse survival.47,48 Subsequent meta-analysis of the literature shows that the difference in death rate is likely negligible49 (Table 19-13). The high rates of local recurrence demonstrated by Ginsberg and others, however, remain a significant concern and continue to restrict the use of limited resection for early-stage lung cancer to the high-risk patient.
| STUDY | STUDY DESIGN | STAGE | NO. OF LIMITED RESECTIONS | NO. OF LOBECTOMIES | REASONS FOR LIMITED RESECTION | SURVIVAL DIFFERENCE |
|---|---|---|---|---|---|---|
| Hoffman and Ransdell (1980)70 | RS | IA | 33 (W) | 40a | Poor cardiopulmonary function and smaller lesions | NS |
| Read et al (1990)71 | RS | IA | 113 (107 S + 6 W) | 131 | ND | NS (CSS) |
| Date et al (1994)72 | MPS | IA | 16 (6 S + 10 W) | 16 | Poor pulmonary function | Lobectomy better |
| Warren and Faber (1994)48 | RS | IA + IB | 66 (S) | 103 | Poor cardiopulmonary function and smaller lesions | Lobectomy better |
| Harpole et al (1995)73 | RS | IA + IB | 75 (W) | 193 | Poor cardiopulmonary function and smaller lesions | NS (CSS) |
| LCSG (1996)47,74 | RCT | IA | 122 (82 S + 40 W) | 125 | Randomization | NS |
| Kodama et al (1997)75 | RS | IA | 46b (W) | 77 | Intentional resection for small lesions | NS |
| Landreneau et al (1997)76 | RS | IA | 102 (W) | 117 | Poor cardiopulmonary function | NS |
| Pastorino et al (1997)77 | RS | IA + IB | 53 (S + W) | 367 | ND | NS |
| Kwiatkowski et al (1998)78 | RS | IA + IB | 58 (S + W) | 186c | ND | Lobectomy better |
| Okada et al (2001)79 | RS | IA ≤2 cm | 70 (S) | 139 | Intentional resection for small lesions ≤2 cm | NS |
| Koike et al (2003)80 | RS | IA ≤2 cm | 74 (60 S + 14 W) | 159 | Intentional resection for small lesions ≤2 cm | NS |
| Campione et al (2004)81 | RS | IA | 21 (S) | 100 | Poor cardiopulmonary function | NS |
| Keenan et al (2004)82 | RS | IA + IB | 54 (S) | 147 | Poor pulmonary function | NS |
With the recent publication of a 20% reduction in lung cancer mortality with screening CT scans in high-risk populations, the topic of limited resection is again the subject of intensive review. Studies investigating anatomic segmentectomy (or extended wedge resection) with hilar and mediastinal lymph node dissection suggest that close attention to the ratio of surgical margin to tumor diameter and a careful assessment of the lymph nodes substantially reduce local recurrence.50,51,52 Recurrence rates were 6.2%, comparable to rates associated with lobectomy, when the margin-to-tumor diameter ratio exceeded 1, compared to 25% if the margin-to-tumor diameter ratio was less than 1.50 In most centers, this requires use of a thoracotomy, although increasing experience with VATS in high-volume centers shows that limited resection is safe and feasible, with perioperative adverse outcomes that are comparable to lobectomy.52,53,54,55
Limited resection, by definition, requires that the patient has sufficient cardiopulmonary reserve to undergo a general anesthesia and loss of at least one pulmonary segment. For the high-risk or nonoperable patient, as determined by experience pulmonary surgeons, tumor ablation techniques have been developed for treatment of early-stage lung cancers.
Current limitations of this approach include the absence of nodal staging, lack of tissue for molecular profiling, chemoresistance, or sensitivity testing, concerns about definitions of locoregional recurrence, and a lack of uniformity across centers. Surgeons typically define locoregional recurrence as tumor growth within the operative field, including resectable lymph nodes, whereas local recurrence after ablation is most commonly defined as tumor growth within the field of treatment. Despite the fact that in-transit or lymph node metastases are present in up to 27% of clinically stage I NSCLCs at resection, any tumor growth outside the field of ablative treatment is not be considered treatment failure.56
Despite these limitations, tumor ablative strategies are increasingly proposed as viable alternatives to surgical resection, even in potentially operable patients.56,58,59,60,61,62 While premature, ablative techniques may ultimately be shown to have efficacy equivalent to lobectomy for the primary treatment of very small peripheral early-stage lung cancers. Multidisciplinary collaboration between thoracic surgery, interventional radiology/pulmonology, and radiation oncology is required to ensure that development of these ablative techniques occurs through properly designed and well-controlled prospective studies and will ensure that patients receive the best available therapy, regardless of whether it is surgical resection or ablative therapy.
The two most commonly applied ablation techniques are radiofrequency ablation and stereotactic body radiotherapy.
Radiofrequency ablation. Radiofrequency ablation is performed using either monopolar or bipolar delivery of electrical current to electrodes placed within the tumor tissue. In lung tumors, the electrodes are typically inserted into the tumor mass under CT guidance. An electrical current is delivered; the current is converted by means of friction into heat, which quickly leads to immediate and irreparable tissue destruction in the tissue surrounding the electrode. The efficacy of radiofrequency ablation for controlling the primary tumor and improving survival in poor operative candidates (either due to significant comorbid diseases precluding general anesthesia or poor pulmonary function excluding lung resection) is safe and feasible for peripheral lung nodules. In tumors <3.5 cm, the rate of radiographic resolution of tumor is up to 80% and cancer-specific survival at 2 years was approximately 90%, indicating excellent local control of the primary site.57,58,59,63 It has become the preferred modality for small peripheral tumors over standard external-beam radiation in centers where the technique is available.
Radiofrequency ablation is an excellent modality for the patient at risk for adverse outcomes with pulmonary resection or for patients who refuse surgery, and surgeons should have an algorithm for determining which patients are optimal for this modality.64,65,66,68,69 (see Fig. 19-24). Target lesions larger than 5 cm, tumor abutting the hilum, associated malignant pleural or pericardial effusion, greater than three lesions in one lung, and the presence of pulmonary hypertension are all contraindications to radiofrequency ablation.64 Proximity to a large vessel is a contraindication not only due to the risk of massive bleeding, but also because large blood vessels act as a heat sink and lethal cellular temperatures are less likely to be achieved. For these patients, stereotactic body radiotherapy may provide local tumor control with less risk of major complications. Combination therapy with either external-beam radiation or stereotactic body radiotherapy is also under investigation.
Stereotactic body radiotherapy.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree