Overview
Lipids, driven by their hydrophobic and hydrophilic portions, form the biological membranes found in all living creatures. These include the cell and nuclear membranes as well as membranes that are part of organelles such as mitochondria and the endoplasmic reticulum (ER). Membranes provide separation of different environments to permit a variety of biological functions. Membranes are not static structures, though, rather they are dynamic and fluid and allow selective movement of ions, energy sources, vitamins and cofactors, and waste. Complex lipids such as cholesterol and sphingolipids both affect the structure of membranes where they are found and are also involved in specific functions.
The variety of lipid and protein molecules, which make up membranes, are responsible for essential functions such as channels and transport across the membrane as well as signaling. Membrane receptors, along with their cytoplasmic partners, transmit signals via steroid hormones. An important type of membrane receptors are G-proteins with intrinsic enzyme activity. The resulting secondary messenger molecules amplify and transmit this signal to various parts of the cytoplasm and nucleus. This ability to transmit a message across a membrane is paramount to not only the normal functions of cells but also their ability to perform specialized functions, which define the human body.
Not surprisingly, lipids and membrane structure and function are important in disease processes as well as treatments. Any deficiencies or problems with lipid synthesis or breakdown lead to a serious disease state and/or death. Modulation of membrane fluidity is, itself, important in membrane functions and, as a result, a variety of diseases. For example, multiple bacteria and viruses are infective and several medications work simply because their hydrophobic nature affects and directly targets lipids and membranes.
Membrane Structure
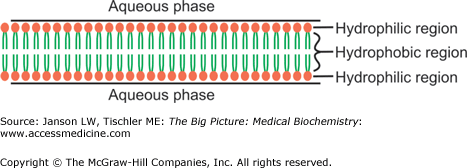
Membranes are composed of lipids arranged in a lipid bilayer, with the hydrophilic glycerol and phosphate “head” groups of the lipid molecules forming the two outside layers and the hydrophobic “tail” groups arranged inside (Figure 8-1).
Figure 8-1.
Simplified Representation of Lipid Bilayer/Membrane. The lipid bilayer is composed of lipid molecules, with hydrophilic head groups (e.g., phosphate) forming the outer surfaces and hydrophobic tails grouped together in the hydrophobic center. See Chapter 3 for more detailed discussion of lipid molecule structures. [Adapted with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
A majority of membrane lipids are phospholipids, usually with 16- or 18-carbon tails, some saturated and some unsaturated. Other types of lipids, including cholesterol, modulate the structure and, therefore, the fluidity of the membrane and, in turn, affect some membrane functions.
Particular lipids are found in specific membranes and are responsible for specific function as summarized below:
- Cardiolipin (Diphosphatidylglycerol)—Several locations including the inner mitochondrial membrane. It has a negative charge and may be involved in (a) decreasing blood clots, (b) stabilizing important respiratory chain enzymes in the mitochondria, (c) moving proteins and cholesterol from the outer to the inner mitochondrial membrane, (d) assisting in the proper folding of mitochondrial proteins (Chapter 9) as a chaperone, and (e) possibly regulating deoxyribonucleic acid (DNA) synthesis.
- Phosphatidylserine (PS)—Located in the inner platelet membrane and, during activation, the exterior platelet membrane. It carries a negative charge and is the primary phospholipid that promotes the anticoagulant protein C pathway, providing feedback inhibition of thrombin formation.
- Phosphatidylethanolamine (PE)—Located in both the interior and exterior of cell membranes. It carries a neutral charge and promotes the anticoagulant protein C pathway, but to a lesser degree than PS.
- Phosphatidylcholine (PC)—Located in the interior and exterior of cell membranes. It carries a neutral charge and promotes the anticoagulant protein C pathway, but to a lesser degree than PS.
- Phosphatidylinositol—Located in the interior and exterior of cell membranes as well as the nuclear membrane. It carries a positive charge and promotes the anticoagulant protein C pathway, but to a lesser degree than PS, and is important in signaling processes for initiation of DNA replication.
Cardiolipin and Disease: Cardiolipin has been suggested to play several important roles in a variety of tissues, and deficiencies in the production and modification of this lipid, not surprisingly, lead to several human disorders. Barth syndrome is an X-linked disease resulting from defects in an enzyme that remodels cardiolipin. The disease results in decreased phosphorylation of adenosine diphosphate molecules, presumably related to cardiolipin’s proposed role in stabilizing respiratory chain enzymes. Changes in the amount or composition of cardiolipin in heart and brain mitochondria may also play a role in diseases such as heart failure, diabetes, Alzheimer’s disease, and Parkinson’s disease. Finally, Trepenoma pallidum, the bacterium responsible for the disease syphilis, produces antibodies against cardiolipin; the biological and disease implications of this are still not well understood. |
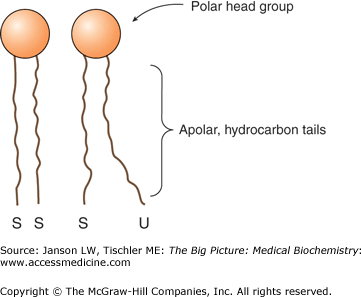
The smaller head groups of phospholipids such as PS and PE are preferred on the inner side of the membrane bilayer. On the other hand, PS is often located on the outside of the bilayer where it can increase adherence to other cells and tissues. A membrane-bound enzyme called “flippase” catalyzes the process of moving particular phospholipid molecules from one side of the bilayer to the other when required. Other phospholipids, because of their head group size and charges as well as the number and location of their tail double bonds, create a more fluid membrane more amenable to areas of cell curvature or cell—cell connections (Figure 8-2).
Figure 8-2.
Membrane Packing of Saturated and Unsaturated Lipid Tails. The lipid bilayer is composed of lipid molecules whose tail groups can be saturated (“S”) or unsaturated (“U”). Saturated tails pack close together, whereas unsaturated tails have a looser packing structure, leading to a more fluid lipid membrane. Increased amounts of saturated or unsaturated lipids can markedly affect membrane fluidity and, therefore, the function of the membrane at that location. [Adapted with permission from Murray RA, et al.: Harper’s Illustrated Biochemistry, 28th edition, McGraw-Hill, 2009.]
Composition of the plasma membrane and, therefore, fluidity has been implicated in several disease processes, including the following:
During stages of infection with malaria, the composition of infected red blood cells is markedly changed to mimic that of the infecting parasite. This may assist in the rupture of red blood cells and the subsequent spread of the parasites that is a hallmark of this disease.
Various bacteria and viruses are believed to change the plasma membrane of target cells to allow the bacteria/virus to fuse and, therefore, infect the modified cells.
Human immunodeficiency virus (HIV) fuses with specific parts of an infected cell’s membrane similar to its own viral membrane in composition and fluidity, allowing new viral particles to emerge and spread to other cells. Research has shown that changing membrane composition of target cells may allow protection from HIV infection.
The parasite Entamoeba histolytica produces proteins that bind to and form holes in human cell membranes, killing these target cells. However, the parasite membrane has a markedly different membrane composition, including different phospholipids and a high level of cholesterol, which protects itself from these deadly proteins.
Membrane composition is markedly changed in the platelets of patients with frequent migraines. Although the mechanism is not known, these changes may be part of the cause of migraine headaches and a better understanding could, therefore, lead to a novel treatment.
Virgin olive oil alters the composition of plasma membranes and affects regulatory proteins in the lipid bilayer, which results in beneficial changes in carbohydrate and lipid metabolism.
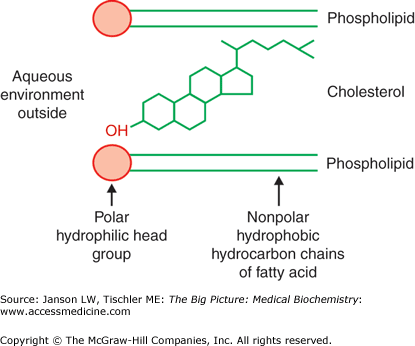
The presence and amount of cholesterol also influence the fluidity of membranes. Cholesterol’s hydroxyl group readily interacts with phospholipid head groups or exterior water molecules and the remaining hydrophobic tetracyclic structure and “tail” also naturally insert inside the lipid bilayer (Figure 8-3). Hydrogen bonding stabilizes these interactions. Depending on the adjacent lipids, cholesterol either increases or decreases the lipid tail packing. Localized membrane “rafts” containing high levels of cholesterol have been noted by researchers and are felt to directly influence not only membrane functions but also the functions of the membrane proteins within them.
Figure 8-3.
Cholesterol Interaction with Membrane Lipids. Illustration of the positioning of cholesterol within the lipid membrane. The hydroxyl group on one end of the molecule prefers to be near the polar, lipid head groups of the external aqueous environment, whereas the remainder of the cholesterol molecule prefers to be within the hydrophobic lipid tails. [Reproduced with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
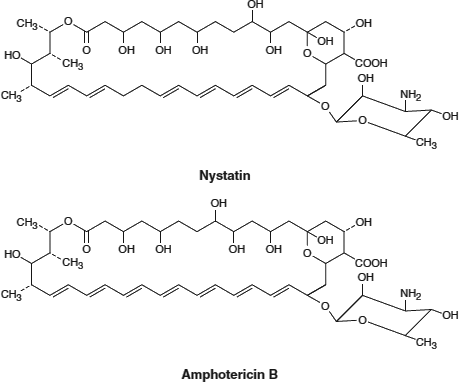
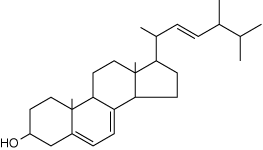
Polyene Antifungals: Antifungal medications including Nystatin and Amphotericin B * (see the figures below) share the structural basis of the polyene molecule (i.e., multiple carbon≕carbon double bonds) opposite an additional carbon chain containing multiple hydroxyl groups (OH). This structure enhances binding to ergosterol, a cholesterol-like, complex lipid found in fungal cell membranes. Binding of these antifungals to ergosterol decreases membrane fluidity and leads to leakage of the inner contents and fungal cell death. Human cell plasma membranes are not affected beacuse they do not contain ergosterol, and the antifungal structure does not bind to cholesterol.
[Adapted with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009.]
[Adapted with permission from Murray RA, et al.: Harper’s Illustrated Biochemistry, 28th edition, McGraw-Hill, 2009.] *Amphotericin B is well known for a variety of serious and even fatal side effects, which are believed to be due to mechanisms other than its effects on fungal membrane fluidity. |
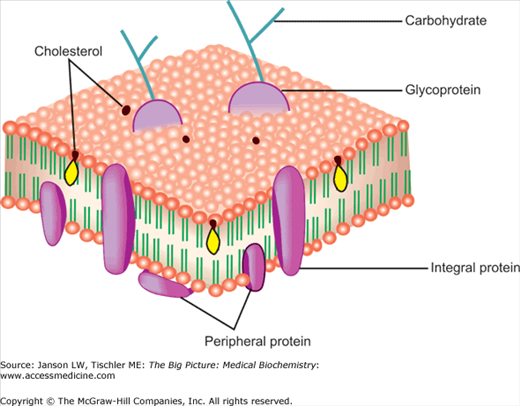
Proteins are the second major part of biological membranes and make up approximately 20%–80% of both the structural and functional components of these membranes. Membrane proteins are classified as peripheral—predominately on the inner or the outer surface of the membrane bilayer—and integral—predominately within the membrane (Figure 8-4). Peripheral membrane proteins are believed to mainly act as anchor points for attachment to external structures (e.g., the extracellular matrix) and internal points (e.g., the actin, microtubule, and intermediate filament cytomatrix; Chapter 12). Integral proteins are usually composed of uncharged, hydrophobic amino acids so they can enter and stay in the hydrophobic environment of the lipid bilayer. Integral proteins serve several functions including channels, “carrier proteins” (transporting molecules through the membrane), or as a signaling protein (changing a portion of their internal cytoplasmic structure in response to a signal at an exposed external part of their structure to activate additional, closely associated molecules, leading to a change of function, e.g., turning on a gene).
Figure 8-4.
Peripheral and Integral Membrane Proteins. The relative positions of peripheral and integral membrane proteins in the lipid bilayer are illustrated. Membrane proteins can also contain carbohydrates resulting in membrane glycoproteins important in membrane signaling and functions. The presence and positioning of cholesterol are also illustrated. [Adapted with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
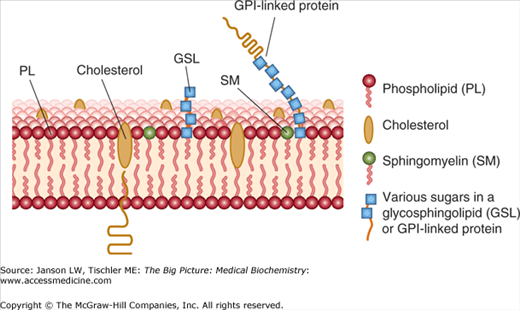
As already illustrated, the membrane content of cholesterol and saturated/unsaturated lipids impacts membrane fluidity. Membrane proteins form connections to the extracellular matrix, the internal cytomatrix, and even other cells that can allow particular proteins and lipids to collect at special areas of a biological membrane. Thus, a cell can orchestrate a particular “area” or “side” where it can receive signals and/or transport particular molecules both in and out and/or other essential functions. These specialized “domains” within membranes are called “lipid rafts” (Figure 8-5). Various examples of these membrane protein functions are discussed below.
Figure 8-5.
Diagram of Lipid Raft. Lipid rafts are somewhat thicker and contain higher amounts of specialty lipids [e.g., sphingomyelin, gangliosides (not shown), saturated phospholipids (non zig-zag tails), and cholesterol]. Membrane proteins can also contain carbohydrates resulting in membrane glycoproteins important in membrane signaling and functions. [Adapted with permission from Murray RA, et al.: Harper’s Illustrated Biochemistry, 28th edition, McGraw-Hill, 2009.]
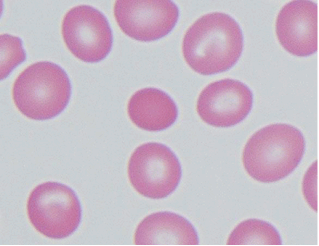
Hemoglobinopathies: Diseases of red blood cells, known collectively as “hemoglobinopathies,” often result from changes in lipid composition and/or defects in proteins associated with the red cell membrane. Elliptocytosis and spherocytosis (see the Figure) are two such diseases that result from defects in spectrin and ankyrin and other red cell membrane proteins important in the stabilization of the normal biconcave shape of red blood cells.
Normal erythrocytes surrounding elliptocyte/spherocyte shown in the center [Courtesy of Dr. Walter Kemp, Montana State Department of Justice] Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|