Cerebral Hemispheres: Diagnosis
SURGICAL/CLINICAL CONSIDERATIONS
Goal of Consultation
Determine whether a lesion should undergo resection (e.g., glioblastoma [GBM]) or diagnostic sampling only to be followed by treatment with chemo- or radiotherapy (e.g., lymphoma)
Allow for proper handling of tissue for ancillary studies (i.e., molecular studies, electron microscopy, microbiologic culture)
Change in Patient Management
Tumors requiring resection may have additional tissue excised to obtain tumor-free margins
If specimens are not adequate for diagnosis &/or ancillary studies, additional biopsies may be performed
Clinical Setting
Patients with neurologic symptoms often require tissue sampling
Patients presenting with symptoms and a focal lesion require diagnosis
New onset of seizures
Localizing signs (e.g., hemiparesis, language difficulty)
Signs and symptoms of increased intracranial pressure
Patients with known systemic illnesses and suspected brain involvement require diagnosis
Metastatic carcinoma
Bone marrow transplant or other immunocompromised states
Patients with diseases that require tissue sampling for ancillary studies but that do not require intraoperative diagnosis
Dementing illness, including Creutzfeldt-Jakob disease: Frozen tissue is saved for molecular analysis, and remaining tissue is treated with formic acid and processed by hand
Vasculitis: Levels on paraffin block are more useful for focal lesions
Epilepsy resections: Orientation of hippocampal resections may be needed
SPECIMEN EVALUATION
Neuroimaging
Imaging findings are very helpful in suggesting most likely diagnosis
Neuroanatomic localization
Signal characteristics
Gross
Usually very few distinctive macroscopic characteristics
Gliomas: Soft, gray-translucent, gelatinous texture
Metastatic carcinoma: Red or tan, gritty consistency
Abscesses: Purulent, sometimes with fibrous wall
Distinguish lesional from normal
Normal: White, homogeneous, soft consistency
Frozen Section
Important not to use entire specimen
Additional tissue may not be available for other studies
A minute portion of specimen is taken for cytologic preparation
Frozen section method
Perch tissue to be frozen on small bead of embedding medium, but do not cover with medium
Freeze quickly with light touch of metal heat extractor or cryospray to avoid ice crystals in tissue
Step section carefully into block to preserve tissue when making slides
In some cases, cytologic preparations without frozen sections may be preferable
Very small specimens
Suspected infectious cases
Specimens with calcifications
Cytologic Preparations
Smear method
Place 1-3 pinhead-sized fragments 1/3 of the way down on glass slide
Use 2nd slide to gently smear tissue
Place immediately in fixative to avoid drying artifact
Touch preparation method
Use for firm/calcified/fibrous lesions
Gently and rapidly touch tissue (held gently in forceps) once to slide surface
If touched multiple times, some of the areas will have air-drying artifact
Place immediately in fixative to avoid drying artifact
Scan entire slide, as lesions may be heterogeneous
MOST COMMON DIAGNOSES
Pilocytic Astrocytoma (WHO Grade I)
Frozen section
Dense areas with fibrillary background, containing Rosenthal fibers and eosinophilic granular bodies, alternating with loose, microcystic regions
Oval nuclei with variable pleomorphism, rare mitoses
Frequent microvascular proliferation, of no prognostic significance
Necrosis rare (suggests alternative diagnosis)
Smear
Clear bipolar cytomorphology
Network of fibers in background
Rosenthal fibers and eosinophilic granular bodies
Knots of microvascular proliferation
Diffuse Astrocytoma (WHO Grade II)
Frozen
Cellularity slightly > normal brain
Cytologic atypia may be mild
Elongated nuclei in infiltrating cells in white matter
Perineuronal satellitosis in cortex
Mitoses very rare
No microvascular proliferation or necrosis
Smear
Fibrillary background clearer than in frozen
Individual cytologically atypical nuclei (hyperchromatic, irregularly shaped, enlarged compared to normal glia)
Difficulties
Findings must correlate with neuroimaging
Diffuse astrocytoma is noncontrast enhancing
Enhancement implies higher grade
Infiltrating edges of high-grade tumors are identical to low grade
Distinction from reactive processes, such as encephalitis, may require special studies (i.e., diagnosis deferred to permanents)
Oligodendroglioma (WHO Grade II)
Frozen section
Uniform, round nuclei
Satellitosis around cortical neurons, subpial tumor cell accumulation
Branching capillary network (“chicken wire” vasculature)
Often, microcalcifications, microcysts
May have microvascular proliferation and rare mitoses
No brisk mitotic activity or necrosis
Smear
Fine fibrillary background
Uniform, round “naked” nuclei (no cytoplasmic processes, in contrast to astrocytomas)
Difficulties
Typical perinuclear halos (“fried eggs”) require formalin fixation, so not present on intraoperative preparations
Often not distinguishable from diffuse astrocytoma
Report as “glioma without anaplastic features”
As for diffuse astrocytomas, must correlate with imaging to make sure sample is not from infiltrating edge of higher grade tumor
Unlike in astrocytomas, microvascular proliferation does not automatically confer higher grade
Oligoastrocytoma (Mixed Glioma) (WHO Grade II)
Features of both diffuse astrocytoma and oligodendroglioma
Diagnosis rarely made on intraoperative consultation
Report of “glioma without anaplastic features” is sufficient
Ependymoma (WHO Grade II)
Frozen section
Variably cellular, with perivascular pseudorosettes, ependymal tubules or canals, and small intracytoplasmic vacuoles (lumina)
Microvascular proliferation of no prognostic significance
Infarct-like necrosis of no prognostic significance
Smear
Glial tumor cells with uniform oval nuclei, often with small nucleoli
Cytoplasmic processes, radially arranged around blood vessels, ± vascular cell proliferation
Occasional intracytoplasmic lumina, as well as cilia and terminal bars (blepharoplasts) in tubules
Difficulties
Though challenging, must distinguish from astrocytoma, as resection is preferred treatment for ependymoma
Anaplastic Astrocytoma (WHO Grade III)
Frozen section and smear
More cellularity and nuclear pleomorphism than grade II astrocytoma
Microvascular proliferation present
Mitoses inconspicuous
No necrosis
Difficulties
May be indistinguishable from anaplastic oligodendroglioma or glioblastoma
Report of “high-grade glioma” is sufficient
Anaplastic Oligodendroglioma (WHO Grade III)
Frozen section and smear
More cellularity and nuclear pleomorphism than grade II oligodendroglioma
Brisk mitotic activity is usual
May have necrosis, microvascular proliferation
Difficulties
May be indistinguishable from anaplastic astrocytoma or glioblastoma
Report of “high-grade glioma” is sufficient
Anaplastic Mixed Oligoastrocytoma (WHO Grade III)
Features of both astrocytoma and oligodendroglioma
Diagnosis rarely made on intraoperative consultation
Report of “high-grade glioma” is sufficient
Anaplastic Ependymoma (WHO Grade III)
Frozen section and smear
Higher cellularity, pleomorphism, and mitoses than in grade II ependymoma
Necrosis prominent
Ependymal tubules or perivascular pseudorosettes may be inconspicuous
Difficulties
Evidence of ependymal differentiation may be scarce, making distinction from anaplastic astrocytoma or glioblastoma challenging
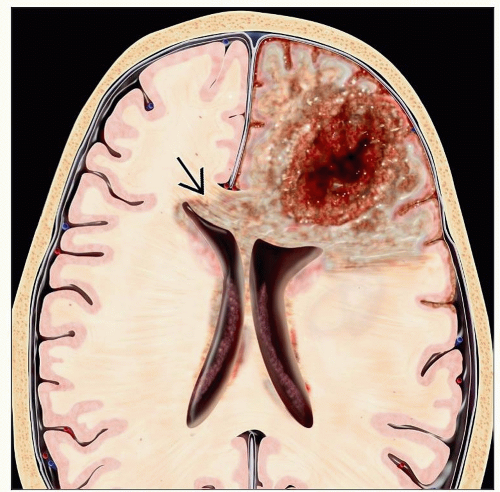
Glioblastoma (GBM) (WHO Grade IV)
Frozen section
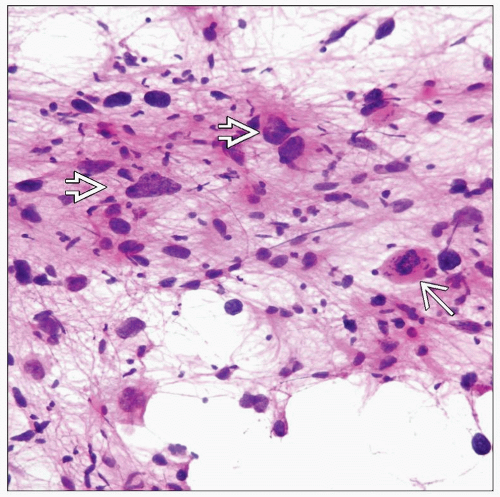
Dense cellularity, pleomorphism, mitotic activity in excess of lower grades
Tumor cells may have spindled, epithelioid, gemistocytic, small cell, &/or giant cells
GBM variants: Gliosarcoma, small cell GBM, giant cell GBM, granular cell GBM
Usually not necessary to distinguish at time of intraoperative consultation
Microvascular proliferation with glomeruloid profiles
Necrosis with peripheral nuclear palisading
Smear
Cytologically malignant cells (hyperchromasia, high N:C ratio, irregular nuclear outline, mitoses)
Coarse fibrillary background
Knotted and blind-ending glomeruloid vessels
Necrosis, sometimes with nuclear debris
Difficulties
Occasionally, epithelioid features mimic carcinoma
If only necrotic tissue received, cannot distinguish from necrotic metastasis or lymphoma, infarct, or inflammatory process
Glioneuronal Tumors
Ganglioglioma
Usually indolent tumor of childhood, arising in temporal lobe
Smear and frozen
Atypical ganglion cells scattered among variably pleomorphic astrocytoma nuclei, fibrillary or myxoid background
Perivascular lymphocytic cuffs
Defer grade, unless obvious anaplasia (mitoses, microvascular proliferation, or necrosis)
Dysembryoplastic neuroepithelial tumor (DNT)
Low-grade, multinodular, cystic tumor of superficial cortex of young patients
Smear and frozen
Small round neurocytic or oligodendrocyte-like nuclei in single-file or nodular growth pattern
Scattered ganglion cells “floating” in microcystic spaces, or myxoid background
May have cortical disorganization and abnormal neuronal cytomorphology (cortical dysplasia) at interface with brain
Central neurocytoma
Low grade, usually arising in ventricles but may be extraventricular
Strictly speaking, a neurocytic tumor but with astrocytic features detectable in some cases
Smear and frozen
Small round neurocytic or oligodendrocyte-like cells, often with perinuclear halos
Fine branching capillary network
May be impossible to distinguish from oligodendroglioma on frozen
Supratentorial Primitive Neuroectodermal Tumors
Smear and frozen: Small blue cells, with high apoptotic and mitotic indices
Frozen: Well- or poorly formed tumor cell rosettes with fibrillary centers
May be difficult to distinguish from small cell GBM or lymphoma on a limited biopsy
Other Primary Neuroepithelial Tumors
Pleomorphic xanthoastrocytoma
Superficial cortical lesion, often with cyst, in young adults
Frozen and smear
Bizarre ganglioid and astrocytic cells, some with foamy cytoplasm
Eosinophilic granular bodies in background
Definitive diagnosis may require ancillary studies (BRAF analysis, immunohistochemistry)
Subependymal giant cell tumor of tuberous sclerosis
Bulky, nodular tumor in floor of lateral ventricle
Usually, patient has stigmata of tuberous sclerosis (cortical tubers, sebaceous hyperplasia, subungual nodules, Lisch nodules, “ash-leaf” spots)
Frozen and smear: Bizarre cytomorphology with large cells having ganglioid and astrocytic features
Pilomyxoid astrocytoma
Large bifrontal tumor of infants
Smear and frozen
Bipolar glial cells in myxoid background
No Rosenthal fibers or eosinophilic granular bodies
Behaves more aggressively than pilocytic astrocytoma
If recognized intraoperatively, a more extensive resection might be considered
Choroid plexus tumors
Papilloma
Frozen: Well-formed papillary structures with benign cuboidal or ciliated epithelium
Smear: Papillary structures well seen
Atypical papilloma
Frozen and smear: More complex configurations of epithelial structures, with nuclear atypia
Choroid plexus carcinoma
Frozen and smear: Very atypical, indistinguishable from metastatic adenocarcinoma
Pineal region tumors
Pineoblastoma: Virtually identical to primitive neuroectodermal tumor (PNET) on smear, frozen section
Pineocytoma: Uniform round neurocytic cells, abundant neuropil, no mitoses
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree