|
Typical Clinical Features |
Microscopic Features |
Ancillary Investigations |
Nodular fasciitis |
Young adults, extremities, head, and neck
Short history—weeks; rarely up to a year
Rapid growth then stops <5 cm
Mostly subcutaneous or occasionally intramuscular
Similar lesions in bladder, spermatic cord, nerve |
Circumscribed or minimally infiltrative
Variably myxoid, cellular, and collagenous areas
Normal mitoses acceptable but not atypia or necrosis
Intravascular variant |
SMA+, desmin±, h-caldesmon−, t(17;22)(p13;q22.1), USP6-MYH9 fusion |
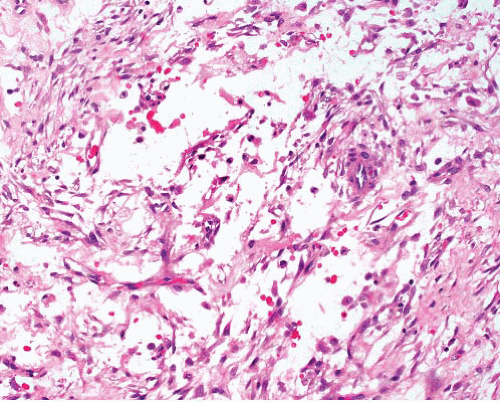
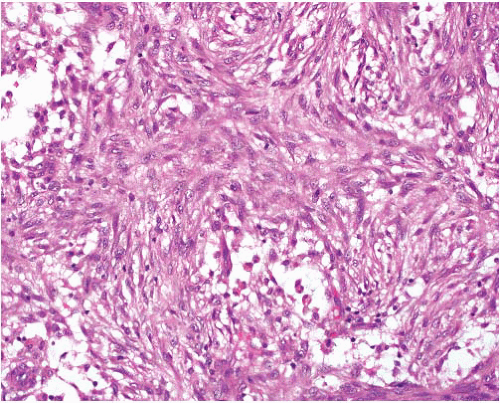
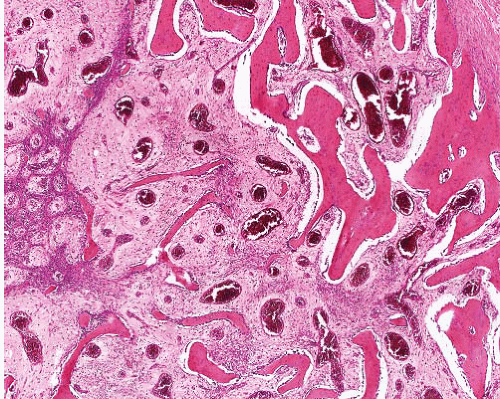
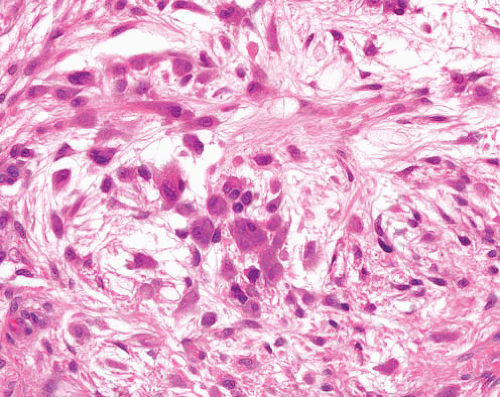
Myofibroma |
Mostly in children, occasionally in adults, in head and neck, extremities, bone
Dermis or subcutis
Deep, visceral multicentric tumors can be fatal |
Circumscribed, occasional central necrosis
Nodules of myofibroblasts, central smaller cells with pericytomatous pattern
Giant cells, ossification, intravascular spread |
SMA+, desmin-negative, h-caldesmon-, S100 protein−, CK− |
Low-grade myofibrosarcoma |
Head and neck sites (including bone), extremities, retroperitoneum
Infiltrative mass, recurs and occasionally metastasizes |
Infiltration of fat and muscle by cellular fascicles of tapered cells with mostly uniform nuclei but focal nuclear atypia
Occasional necrosis
Occasional myxoid change focally |
SMA+, desmin±, h-caldesmon− |
Leiomyosarcoma |
Slowly growing
Tumors confined to dermis can recur but rarely metastasize.
Those in subcutis have increased metastatic potential
Note: leiomyoma occurs in skin but is very rare in deeper tissue especially in males. |
Fascicles intersect at right angles.
Nontapered cells with eosinophilic cytoplasm and blunt-ended nuclei
Nuclear atypia, mitotic activity, and focal necrosis
Inflammatory variant has lymphocytic infiltrate, foamy macrophages, psammoma bodies |
SMA+, desmin+, h-caldesmon+ |
Low-grade myxofibrosarcoma |
Limbs, older adults, subcutaneous, fascial, or deeper
Recurs, often with grade progression |
Multinodular, myxoid, spindle cells with hyperchromatic nuclei and variable nuclear atypia, pleomorphic cellular areas |
CD34±
Lacks myoid antigens except in pleomorphic areas |
Inflammatory myofibroblastic tumor |
Mesenteric, retroperitoneal, other sites |
Fascicular, myxoid, and sclerosing patterns
Mostly bland myofibroblasts, occasional atypical polygonal “ganglion-like” cells
Marked inflammation, notably with plasma cells often in aggregates |
SMA+, ALK+ 55%, ALK gene rearrangements |
Proliferative fasciitis |
Middle-aged adults, extremities, trunk
Rapid growth of firm subcutaneous nodule |
Polygonal cells with prominent nucleoli in fasciitis-like background
No atypia, no acute inflammation |
SMA+ in spindle cells, polygonal cells negative for CD34, S100 protein, desmin, CK, EMA |
Myxoinflammatory fibroblastic sarcoma |
Mostly extremities, adults
Cutaneous or subcutaneous, can involve tendons |
Multinodular myxoid foci with vacuolated fibroblasts, cellular areas with Reed-Sternberg-like and atypical mononuclear cells
Eosinophils, plasma cells, hemosiderin |
CD34+ in some
t(1;10)(p22;q24), TFGBR3 and MGEA5 rearrangements |
Angiofibroma of soft tissue |
Superficial or deep, lower extremity, near joints, female predominance |
Circumscribed, bland spindle cells, prominent thin-walled vessels and ectatic spaces |
EMA+, CD34±, SMA±, desmin±
t(5;8)(p15;q13), AHRR–NCOA2 fusion |
Solitary fibrous tumor |
Subcutaneous or deep, circumscribed tumor
Can occur in any location, body cavity, or organ |
Circumscribed, not usually encapsulated
Distinct cellular and fibrous areas, focal myxoid stroma, patternless short spindle cells
Hemangiopericytomatous pattern focally
Malignant variant has hypercellularity, mitoses >4 per 10 hpf, necrosis
Dedifferentiated solitary fibrous tumor has pleomorphic component ± heterologous elements |
STAT6+, CD34+, bcl-2+, CD99 +, GRIA2+ |
Fibromatosis—superficial |
Palm, sole, penis; infiltrative plaque |
Variably cellular nodules, cells spindled or ovoid, evenly dispersed in dense collagen, mast cells
Normal mitoses acceptable but not atypia or necrosis |
SMA+, nuclear betacatenin + occasionally, CD34− |
Fibromatosis—desmoid type |
Deep, limbs, head and neck, body cavities |
Parallel-aligned myofibroblasts evenly dispersed in collagen, slit-like and thick-walled vessels, mast cells
Normal mitoses acceptable but not atypia or necrosis |
SMA+, nuclear betacatenin+, CD34− |
Fibrous hamartoma of infancy |
M > F, most appear in first 2 years of life
Axilla, trunk, inguinogenital region |
Infiltrative tumor with variable mixture of (1) cellular fibrous bands containing small stellate or ovoid cells, (2) neurofibromalike spindle cell component, (3) mature fat
Lipofibromatosis has morphologic similarities. |
SMA+ focally, rarely desmin+ or CD34+. S100 protein− |
Cellular fibrous histiocytoma |
Dermal nodule of varying size, extremities, trunk
Young adults, M > F |
Fascicles of tapered spindle cells in dermis, radial extensions into subcutis
Mitoses variable
Peripheral collagen bundles, rare foamy, and giant cells
Central necrosis in some
Atypical variant has scattered enlarged hyperchromatic nuclei or multinucleation in background of typical cells. |
SMA+, desmin rarely+, h-caldesmon rarely+ |
Deep benign fibrous histiocytoma |
Young adults, M > F, extremities, head and neck
Subcutaneous mass can reach large size.
Can recur if incompletely excised, very rarely metastasizes |
Circumscribed, often with fibrous capsule
Fascicles and storiform whorls of short spindle cells with variable mitotic activity
Lymphocyte sprinkling, multinucleated and foamy cells, hemosiderin, focal fibrosis, focal hemangiopericytomatous pattern
Rarely atypia or necrosis |
CD34± (40%), SMA± (38%), desmin+ occasionally, S100 protein− |