Carotid Surgery: Interposition/Endarterectomy (Including Eversion)/Ligation
Vinit N. Varu
Wei Zhou
DEFINITION
Stroke is the leading cause of disability in the United States and Western Europe and the third leading cause of death behind coronary artery disease and cancer.
Pivotal studies have shown the efficacy of carotid endarterectomy (CEA) in stroke prevention in both symptomatic and asymptomatic patients with internal carotid artery (ICA) stenosis versus medical therapy alone.1,2
CEA is defined as the surgical excision of atherosclerotic lesions of the intima and tunica media of the carotid artery.
Occasionally, ICA ligation and/or interposition bypass may be indicated for stroke prevention.
PATIENT HISTORY AND PHYSICAL FINDINGS
Patients may be entirely asymptomatic and still benefit from carotid intervention to prevent long-term stroke. In the United States, most CEA procedures are performed on asymptomatic patients. Symptoms of cerebroembolic disease originating from the carotid bifurcation, when present, may include dysarthria, dysphasia, aphasia, hemiparesis, or hemisensory deficit or amaurosis fugax. Symptoms that resolve within 24 hours are defined as transient ischemic attacks (TIAs) regardless of severity; symptoms that persist past the first day constitute a stroke.
For patients at risk for cerebroembolic disease, a thorough vascular history is obtained including modifiable risk factors such as smoking, hyperlipidemia, hypertension, and diabetes management. Prior to surgery, single-agent antiplatelet therapy is initiated and continued indefinitely following intervention. Blood pressure control at or below 140 mmHg systolic and 90 mmHg diastolic is the single most important medical intervention to reduce stroke risk.3 Sufficient β-blockade to stabilize resting heart rate at 60 bpm is also instituted prior to surgery to limit perioperative myocardial oxygen demand unless contraindicated.4
Cervical auscultation is performed in both the supraclavicular and mandibular regions. Bruits appreciated at the mandibular angle usually indicate ICA or bifurcation disease. More proximal bruits may indicate common carotid artery (CCA) disease or radiating heart sounds.
A full neurologic assessment including mental status, speech, facial symmetry, and extremity strength must be obtained and documented prior to surgery.
IMAGING AND OTHER DIAGNOSTIC STUDIES
All patients exhibiting symptoms of carotid territory ischemia need appropriate vascular imaging studies. Screening is not recommended to detect asymptomatic disease in the general population; patients with appropriate risk factors, or those with a bruit on physical exam should be evaluated when clinical circumstances warrant.
Carotid duplex ultrasound provides a reliable and accurate noninvasive tool to identify predicted stenosis and is the initial diagnostic study of choice. Peak systolic velocity (PSV) higher than 125 cm per second predicts angiographic stenosis more than 50% and higher than 230 cm per second predicts more than 70% stenosis. However, a combination of PSV, end diastolic velocity, and the PSV ratio of ICA to CCA is more accurate in estimating significant carotid stenosis. In general, end diastolic velocity higher than100 cm per second correlates to more than 80% carotid stenosis.
When duplex imaging is not definitive, as is the case in the setting of extensive carotid bifurcation calcification, additional cross-sectional imaging (computed tomography angiography [CTA] or magnetic resonance angiography [MRA]) may be necessary to quantify the degree of stenosis. When accurate velocity information is obtainable, duplex imaging provides the most accurate and physiologically relevant estimates of percent diameter reduction.
SURGICAL MANAGEMENT
Indications
Endarterectomy
The Society for Vascular Surgery recommends that neurologically symptomatic patients with greater than 50% stenosis or asymptomatic patients with greater than 60% stenosis should be offered CEA to reduce risk of recurrent or initial stroke, respectively. Endarterectomy is appropriate for patients with at least a 3- to 5-year life expectancy with perioperative stroke/death rates less than 3%. In all other circumstances, optimal medical therapy is preferred.5
Surgical endarterectomy is the procedure of choice for good-risk surgical patients with normal cervical anatomy. For selected high-risk patients, such as those with tracheal stoma, previously radiated neck, prior cranial nerve injury, or lesions proximal to the clavicle or distal to C2 vertebral body, transcatheter angioplasty and stenting is generally the preferred approach.5 Indications and technical guidelines for carotid angioplasty and stenting procedures are discussed in Part 6, Chapter 4.
Carotid Artery Interposition Bypass
Reconstruction for extensive bifurcation disease, injury to the bifurcation during endarterectomy, or aggressive restenosis following previous intervention (endarterectomy or stent placement) is best accomplished by carotid resection
and interposition grafting. Other indications include the following:
Significant diffuse CCA and ICA disease
Radiation-induced stenosis or other forms of arteritis involving long arterial segments
Aneurysms (degenerative or traumatic) and invasive carotid body tumors.
Ligation
Ligation and resection of the proximal ICA may be indicated in the setting of carotid stump syndrome, when persistent distal embolization from the “cul-de-sac” of the occluded ICA may reflux into collateral pathways, such as through the ophthalmic artery into the distal ICA.
Preoperative Planning
Similar outcomes are achieved with general anesthesia or regional anesthesia.
Use of shunt during CEA is dependent on operator preference. Most surgeons either shunt selectively or use a shunt for all cases. Some surgeons never shunt.6 Surgeons should develop the methods they feel most comfortable with to optimize outcome. Objective measures that may influence shunt usage include stump pressure measurement, electroencephalographic monitoring, and transcranial Doppler assessment. Data supporting use of these adjuvants is inconsistent, and none is considered standard of care nationally.
Optimal neck extension is obtained by placing a towel or gel roll behind the scapula. The head is rotated contralateral to the operative side. In older patients, often with limited neck movement or prior cervical fusions, padding and shay positioning must be sufficient to support the neck to prevent hyperextension injury. The chin, angle of the mandible, lower earlobe, and sternal angle are prepped and preliminarily draped within the operative field. The bed itself can be flexed with the head in relative extension to aid in positioning (FIG 1).
Arterial blood pressure monitoring is necessary for optimal anesthetic management. Bladder catheterization is performed if the procedure is expected to extend beyond 2 hours. If endarterectomy is performed with regional anesthesia, an audible squeeze device is placed in the patient’s contralateral hand for indirect neurologic monitoring. Preoperative antibiotics are administered routinely.
Aspirin therapy is initiated well in advance of surgery and continued throughout the perioperative period. Evidence suggests that statin therapy, initiated preoperatively, reduces postoperative neurologic events and mortality.7
TECHNIQUES
CAROTID ENDARTERECTOMY— PATCH ANGIOPLASTY
Incision
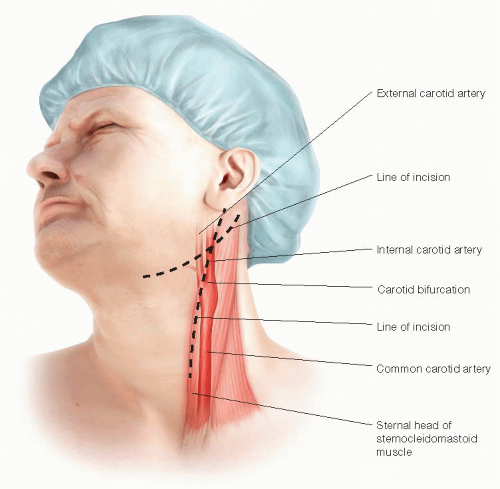
The skin incision is optimally placed along the anterior border of the sternocleidomastoid muscle. This should be curved posterolaterally near the angle of the mandible to avoid dissection into the parotid gland.
Alternatively, a more transverse incision can be made at the level of the carotid bifurcation. Although providing an improved cosmetic result, exposure of the distal ICA may be compromised with this approach (FIG 2).
Carotid Exposure and Control
As the incision is extended through the platysma muscle, the anterior border of the sternocleidomastoid muscle is visualized and retracted posterolaterally. The greater auricular nerve should be identified and protected at the superior extent of the incision.
Following fascial incision, the facial vein is identified and securely ligated. This vein usually transverses the CCA near the bifurcation. Failure to adequately secure this vein may lead to bleeding and airway compromise during postoperative cough spells or Valsalva maneuvers.
Within the carotid sheath, the vagus nerve usually extends posterior to, and parallel with, the artery and vein. However, this position relative to the other contents of the carotid sheath may vary, and the vagus should always be identified and protected in the course of the dissection. The ansa cervicalis nerve is commonly much smaller than the vagus and runs anterior to the carotid bifurcation. When completely isolated, the proximal ansa arises from the ipsilateral hypoglossal (XII) cranial nerve. The ansa cervicalis can be divided to improve exposure if necessary or mobilized sufficiently to be gently retracted out of the operative field.
The CCA is circumferentially dissected from surrounding structures in sufficient length to provide adequate exposure for proximal clamping and control. The CCA is
optimally controlled by placement of an appropriately sized, atraumatic vascular clamp such as a Gregory profunda clamp. The ratchet should be engaged only to the minimal amount necessary to control bleeding to prevent intimal injury and dissection at the site of clamp placement.
Following common carotid control, the dissection is extended cranially and posteriorly along the posterolateral border of the ICA. Development of the dissection plane posterolaterally along the proximal ICA minimizes risk of hypoglossal nerve injury. This dissection is also performed with minimal displacement and instrumentation of the ICA to reduce intraoperative embolization risk (FIG 3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree