Calcineurin Inhibitor Toxicity
Shane M. Meehan, MBBCh
Key Facts
Terminology
Kidney dysfunction attributable to injury from calcineurin inhibitor immunosuppressive therapy
Etiology/Pathogenesis
Transplanted and native kidneys affected by CIT
CIT is dose related
Functional CIT: Reversible acute renal dysfunction associated with afferent arteriolar vasoconstriction
Structural CIT: Tubular and vascular CIT
Clinical Issues
Acute or chronic elevation of serum creatinine
Elevated blood or serum levels of CI
Correlation of structural tissue injury and blood levels is not strong
Microscopic Pathology
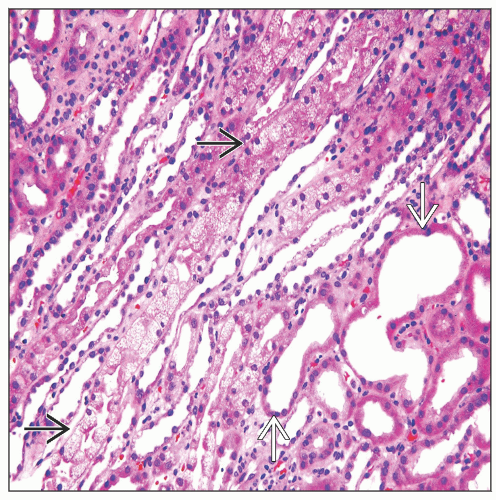
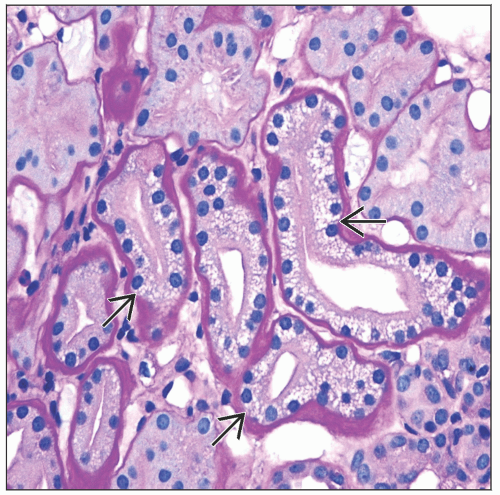
Tubular toxicity: Acute tubular injury with focal isometric vacuolization of proximal tubular segments
Acute arteriolopathy: Smooth muscle loss and expansion of intima and media by loose matrix
Thrombotic microangiopathy (TMA)
Chronic arteriolopathy: Nodular medial hyalinization
Diagnostic Checklist
Exposure to CI is a sine qua non for diagnosis
Absence of pathologic lesions in kidney biopsy does not exclude CIT
Observation of combined tubulopathy and vasculopathy increases diagnostic certainty
TERMINOLOGY
Abbreviations
Calcineurin inhibitor toxicity (CIT)
Synonyms
Cyclosporine toxicity, cyclosporine A (CsA) toxicity, tacrolimus toxicity, FK506 toxicity
Definitions
Acute or chronic kidney dysfunction attributable to direct injury from calcineurin inhibitor drugs
ETIOLOGY/PATHOGENESIS
Types of CIT
Functional CIT: Reversible acute renal dysfunction associated with afferent arteriolar vasoconstriction
Structural CIT: Characterized by cellular injury and matrix remodeling
Tubular CIT: Acute tubular injury with vacuolization of epithelium
Vascular CIT: Direct toxic injury to endothelium of arterioles and glomeruli, as well as to smooth muscle of arterioles
May manifest as thrombotic microangiopathy or chronic hyaline arteriolopathy
Tubular and vascular toxicity typically coexist
Native and transplanted kidneys are affected by CIT; histologic manifestations are similar
Mechanisms
Histologic lesions are dose related
Acute CIT with markedly elevated blood levels of CI
Chronic CIT with long-term exposure to CI
May also be dose-independent susceptibility factors
CsA and tacrolimus bind intracellular receptors called immunophilins
Immunophilin/CI complexes bind, inhibit calcineurin
Calcineurin is T-cell activator via nuclear factors of activated T cells (NFAT)
NFAT activate transcription of inflammatory mediators like interleukin-2, interferon γ, and tumor necrosis factor α
Immunosuppressive potency and renal toxicity of CI are pharmacologically inseparable
Nephrotoxic effects of CsA & tacrolimus are identical
CIT affects endothelium, vascular smooth muscle, and tubular epithelium
Endothelium: Increased thromboxane A2, endothelin-1, superoxide and peroxynitrite, decreased prostaglandin and prostacyclin, apoptosis, necrosis
Smooth muscle: Vacuolization, necrosis, apoptosis, hyalinization
Tubular epithelium: Vacuolization, megamitochondria, calcification, necrosis
CLINICAL ISSUES
Epidemiology
Incidence
In kidney transplants
Thrombotic microangiopathy (TMA) attributable to CI toxicity in 2-5%
Chronic CIT in 60-70% at 2 years and > 90% at 10 years
Presentation
Acute or chronic elevation of serum creatinine
CIT may arise at any time after initiation of therapy
Elevated trough blood levels of CI may confirm diagnosis, but correlation of structural tissue injury and blood level is not strong
TMA may be localized to kidney (40%) or may be systemic
Treatment
Dose reduction or cessation of CI therapy
Prognosis
Acute CIT is typically reversible and associated with resolution of histologic changes
Chronic CIT is less likely to be reversible; however, resolution of arteriolopathy has been observed with cessation of CI
MICROSCOPIC PATHOLOGY
Histologic Features
Functional CIT
No morphologic tissue injury by definition
Acute tubular CIT
Focal proximal tubular epithelial isometric vacuolization affecting straight more than convoluted segments
Vacuolar changes may be accompanied by acute tubular injury
Proximal tubules may have large eosinophilic cytoplasmic granules corresponding to megamitochondria (CsA toxicity) or lysosomes (tacrolimus toxicity)
Tubular calcium phosphate deposition
Chronic tubular CIT
Cortical tubular atrophy and interstitial fibrosis in a striped pattern (striped fibrosis)
Characterized by radial fibrosis affecting cortex with intervening nonscarred parenchyma
May arise as result of chronic ischemia from arteriolopathy in addition to direct tubular toxicity
Tubular CIT is typically accompanied by vascular CIT, which may be very focal
Vascular CIT
Vasculopathies of CIT tend to be focal and mainly affect arterioles
Acute CIT has spectrum of severity from acute arteriolopathy to TMA
Chronic CIT is characterized by hyalinization of arterioles
Acute and chronic vasculopathy may be evident in same biopsy
Acute arteriolopathy
Loss of definition of smooth muscle cells
Myocyte cytoplasmic vacuolization and dropout from necrosis or apoptosis
Medial swelling and thickening with separation of myocytes
Clear or basophilic medial or intimal loose matrix accumulation
Intimal or medial platelet insudates may be identifiable by immunohistochemistry for CD61
Thrombotic microangiopathy (TMA)
Arteriolar thrombi
Arteriolar intimal and medial fibrinoid exudate with erythrocytolysis
Obliterative arteriolopathy: Stenosis, intimal and medial interstitial swelling, and hypercellularity (“onion skinning”)
Arteries may have intimal myxoid thickening in TMA
Chronic arteriolopathy
Early lesions: Hyaline replacement of individual medial smooth muscle cells
Later nodular hyalinization becomes more prominent in the outer media; imparting an eosinophilic, PAS-positive “beaded necklace” appearance
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree