Cadaveric Donor Nephrectomy and Renal Transplantation
Daniel A. Katz
Rajesh Shetty
In this chapter, harvesting of kidneys and renal transplantation are described as a means of illustrating the anatomy of the retroperitoneum. The en bloc nephrectomy specimen consists of a segment of aorta, a segment of vena cava, the kidneys and their vessels, the ureters, and a generous amount of perinephric tissue, including the adrenal glands.
SCORE™, the Surgical Council on Resident Education, classified en bloc abdominal organ retrieval and kidney transplant as “COMPLEX” procedures.
STEPS IN PROCEDURE
Cadaveric Donor Nephrectomy
Longitudinal midline incision from suprasternal notch to symphysis pubis
Median sternotomy
Chest and abdominal teams work simultaneously
Kidneys dissected free and easily removed after harvest of liver and pancreas
Dissect terminal aorta free, divide inferior mesenteric artery and place two umbilical tapes around aorta
Isolate supraceliac aorta
Heparinize
Insert aortic cannula into terminal aorta
Clamp supraceliac aorta
Place vent in vena cava, usually just above the diaphragm
Perfuse kidneys with 2 L of cold preservation solution
Identify both ureters and divide near the bladder
Tag ureters
Mobilize kidneys medially with Gerota fascia
Divide the aorta and vena cava distally, at the level of the aortic cannula insertion
Mobilize en bloc specimen cephalad while dividing prevertebral fascia
Remove en bloc specimen and complete preparation with separation of kidneys on back table
Renal Transplantation
Foley catheter, instill neomycin solution into bladder
Curvilinear Gibson type pelvic incision
Divide inferior epigastric vessels
Extraperitoneal dissection to expose external iliac artery and vein
Venous anastomosis to external iliac vein
Arterial anastomosis (generally with cuff of aorta) to external iliac artery
Ureteroneocystostomy to dome of bladder
Expose bladder mucosa
Anastomose ureter to bladder mucosa
Close second layer of bladder wall over ureter to create muscular tunnel
Obtain hemostasis and close incision in layers
HALLMARK ANATOMIC COMPLICATIONS
Devascularization of ureter
LIST OF STRUCTURES
Kidneys
Renal artery and vein
Ureter
Bladder
Gerota fascia
Gonadal artery and vein
Adrenal (Suprarenal) Gland
Right adrenal (suprarenal) vein
Aorta
Celiac artery
Superior mesenteric artery
Inferior mesenteric artery (and vein)
Common iliac artery (and vein)
Internal iliac artery (and vein)
External iliac artery (and vein)
Diaphragm
Left and right crura
Inferior phrenic artery
Stomach
Duodenum
Pancreas
Spleen
Colon
Cadaveric Donor Nephrectomy
Cadaveric Donor Nephrectomy: Incision and Exposure of the Chest and Abdomen (Figs. 109.1 and 109.2)
Technical and Anatomic Points
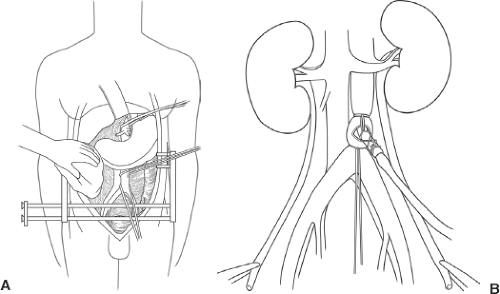
Donor and recipient blood types are confirmed. Time-out is performed according to current UNOS guidelines. After consent is achieved, the donor is placed on the operating table in the supine position and ventilated with 100% oxygen. Exposure for organ harvesting is provided by a longitudinal midline incision that extends from the suprasternal notch to the symphysis pubis (Fig. 109.1A). This incision provides sufficient exposure of and access to the heart, lungs, and abdominal viscera.
The sternum is split with an electric saw or a Lebsche knife; hemostasis of the cut surface is achieved with electrocautery
and bone wax. A sternal retractor with spikes is placed in the lower third of the sternum and spread laterally. A special large Balfour retractor with teeth is used for the abdomen (Fig. 109.1B). The heart and liver teams usually start the dissection simultaneously. The kidneys can be dissected free and easily removed after flushing of the graft and the liver and pancreas are removed.
and bone wax. A sternal retractor with spikes is placed in the lower third of the sternum and spread laterally. A special large Balfour retractor with teeth is used for the abdomen (Fig. 109.1B). The heart and liver teams usually start the dissection simultaneously. The kidneys can be dissected free and easily removed after flushing of the graft and the liver and pancreas are removed.
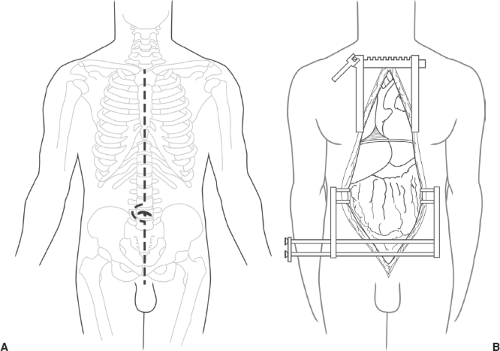 Figure 109.1 A: Cadaveric donor nephrectomy: Incision. B: Placement of retractors and initial exposure. |
Preparation and Flushing of the Graft (Fig. 109.2)
Technical Points
Generally, left and right medial visceral rotations will already have been completed by teams procuring liver, pancreas, or bowel. However, if not done, Mattox maneuver and Kocher–Cattell maneuver at this stage (prior to heparinization) facilitate kidney dissection later in the procedure. Dissection to expose the left renal vein insertion into the inferior vena cava is desirable. Dissect the terminal aorta free. Divide the inferior mesenteric artery between ligatures and place two umbilical tapes around the terminal aorta (Fig. 109.2A). As a last step in the preparatory dissection, encircle the supraceliac aorta with an umbilical tape after separating the muscle fibers of the diaphragmatic crura longitudinally in a blunt fashion and incising the preaortic fascia. This step is omitted if the heart is not procured because the descending aorta can be cross-clamped in the left pleural cavity without preliminary dissection.
Heparin at a dose of 300 to 500 U/kg should be administered intravenously 3 minutes before cross-clamping. For a nonheart-beating donor, the dosage of heparin should be doubled. Tie the terminal aorta with an umbilical tape. Insert the aortic cannula into the terminal aorta (Fig. 109.2B) while controlling the aorta with the left hand proximal to the aortotomy. Position the tip of the aortic cannula below the renal artery to avoid blocking perfusion of renal artery. Secure the aortic cannula with the other, more proximal umbilical tape.
Apply a vascular clamp on the supraceliac aorta and perfuse the kidneys through the terminal aortic cannula with 2 L of preservation solution. At the same time, vent the supradiaphragmatic vena cava, to prevent venous engorgement and to drain flush solution from the operative field. Apply slush to the abdominal and thoracic cavities to cool the organs.
Anatomic Points
The paracolic gutters lie lateral to the colon and are limited medially by the retroperitoneal colon (ascending or descending) and its serosal covering, which became fused with parietal peritoneum during development. The white line of Toldt, visible in the angle between the parietal peritoneum and the lateral colon, marks the location of the fusion plane between original serosa or mesocolon and parietal peritoneum. Dissection in the fusion plane, from colon to midline, results in no damage to structures and minimal blood loss.
The gonadal vessels are the first major retroperitoneal structures encountered just superior to the pelvic brim. The right gonadal vein (a tributary of the inferior vena cava) and artery (an anterolateral branch of the aorta slightly inferior to the renal arteries) should be encountered as they cross the external iliac vessels somewhat lateral to the ureter. The ureter is just medial to these vessels.
More superiorly, Gerota fascia (enclosing the right kidney, suprarenal gland, and perirenal fat) will be exposed as the hepatic flexure is mobilized. Further medial mobilization of the colon will expose the C-loop of the duodenum, encompassing the head of the pancreas. These retroperitoneal structures are in direct contact with the anterior surface of Gerota fascia. Further
medial mobilization of the right colon, terminal ileum, duodenum, and head of the pancreas will expose the entire infrahepatic inferior vena cava lying to the right of the midline.
medial mobilization of the right colon, terminal ileum, duodenum, and head of the pancreas will expose the entire infrahepatic inferior vena cava lying to the right of the midline.
Encirclement of the distal aorta, just proximal to its bifurcation, is aided by lateral retraction of the inferior mesenteric artery because this parallels, to the left, the distal aorta. Skeletonization of the distal aorta; however, should be done with some care to avoid inadvertent laceration of the left fourth lumbar vein, which passes to the left posterior to the aorta.
As the abdominal aorta is skeletonized from inferior to superior, the first structure to be encountered should be the inferior mesenteric artery. More superiorly, again from the anterior surface of the aorta, small gonadal arteries can be identified. These may arise as a common trunk, separately and at the same or different levels, or they may arise from a renal or suprarenal artery. The next structure to be encountered should be the left renal vein, which typically crosses the anterior surface of the aorta just inferior to the origins of the superior mesenteric artery and left renal artery. However, retroaortic left renal veins or circumaortic left renal veins do occur, as commonly as 6% of the time. The superior mesenteric artery usually originates about 1 cm distal to the celiac artery; however, both of these major arteries can arise from a common trunk. Rarely, one or more of the three celiac artery branches (left gastric, splenic, or common hepatic) arise independently from the aorta and the procurement surgeon needs to be aware of these anatomic variations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree