Anatomic constraints for endovascular management of abdominal aortic aneurysms include the presence of short or angulated surgical necks and aneurysmal degeneration of the origins of the visceral arteries. Fenestrated and branched endografts were introduced to enable minimally invasive repair of complex juxta- and suprarenal aortic aneurysms.1 These devices incorporate reinforced fenestrations or directional branches, permitting incorporation of visceral artery origins into the proximal endograft seal zone without compromising end-organ perfusion or aneurysm exclusion.2 This chapter summarizes the technical features of endovascular aneurysm repair using fenestrated and branched stent grafts for pararenal and thoracoabdominal aortic aneurysms.
The term fenestrated repair refers to deployment of an endograft featuring custom orifices created and reinforced at precise locations around the aortic perimeter to enable branch artery access, cannulation, and placement of a bridging stent graft in the course of aneurysm exclusion. Fenestration sites are created from patient-specific cross-sectional image data to enable exclusion of aneurysms with short or angled infrarenal necks. In most circumstances, the target arteries (e.g., renal or mesenteric) must arise from normal aorta to enable fenestrated repair. As a rule, fenestrations must be able to deploy flush with the aortic wall to ensure adequate aneurysm exclusion. “Alignment” stents (covered or uncovered, depending on individual patient circumstance) are deployed as needed to prevent target artery malperfusion as a consequence of misalignment between the fenestration and target artery orifice.
Branched repair refers to endovascular aneurysm exclusion employing covered stents to directly connect the main lumen of the endograft to the target visceral artery. These devices enable repair of aneurysms involving or extending proximal to the origins of the renal or visceral vessels (e.g., type IV thoracoabdominal aortic aneurysms [TAAAs]). Of necessity, some distance must be present between the main body of the endograft at full deployment and the aortic wall at the target visceral artery orifice. Branched stent grafts are currently available in two distinct configurations:
Fenestrated branches arise from reinforced fenestrations bridged by balloon-expandable covered stents.
Directional or cuffed branch devices feature appended fabric cuffs, precisely located to enable straight, helical, down- or up-going guidewire egress, target vessel cannulation, and deployment of bridging covered stents. Self-expanding flexible nitinol stents are usually employed for this purpose.
Most aneurysms are degenerative (previously characterized as “atherosclerotic,” based on a similar, although not identical, causal risk factor profile).
Other relevant etiologies include infection (e.g., mycotic aneurysms), inflammation (e.g., inflammatory aneurysm or aortitis), development of penetrating ulcers or asymmetric saccular enlargement, and related aortic pathologies (dissection or intramural hematoma).
Most patients’ aneurysms do not prompt symptoms prior to catastrophic rupture and are diagnosed incidentally or during screening. Indications for repair are size greater than 5.5 cm for males and greater than 5 cm for females or enlargement greater than 5 mm in 6 months.3
In approximately 5% to 10% of patients, aneurysms induce periaortic inflammation and resultant retroperitoneal fibrosis involving adjacent structures, including the duodenal and ureters.4 These patients may present with abdominal or back pain, fatigue, malaise, or low-grade fever even at relatively small diameters. Commonly, these aneurysms also enlarge at accelerated and unpredictable rates. Other uncommon presentations of abdominal aortic aneurysm disease include the presence of distal embolization with “blue toe syndrome,” congestive heart failure from aortocaval fistulae, or gastrointestinal bleeding from primary aortoenteric fistulae.
A comprehensive history should be obtained to fully appreciate the potential natural history of each patient’s disease, including a comprehensive assessment of cardiovascular risk factors, current smoking habits, and a family history of aneurysmal disease or connective tissue disorders.
Evaluation of perioperative clinical risk emphasizes cardiac, pulmonary, and renal functional status and reserve, including baseline laboratory testing, noninvasive cardiac stress testing, pulmonary function assessment, and carotid duplex ultrasonography when indicated.
Preprocedural aortic imaging studies provide fundamental and necessary guidance for endovascular repair strategies of all types. Aneurysm morphology is best analyzed through acquisition of high-resolution computed tomography angiography (CTA) datasets.5 CTA with submillimeter slice acquisition is recommended for optimal acquisition, allowing three-dimensional reformatting techniques, maximum intensity projections, and volume rendering.
Stent grafts are currently custom-made to conform to patient anatomy, based on estimates of longitudinal distance, axial clock position, arc lengths, and angles derived from centerline of flow measurements.
Anatomic limitations to be considered include difficult iliac access, excessive aortic tortuosity, visceral artery occlusive disease, and anatomic variants including multiple accessory renal arteries or early renal branch bifurcation.
Device planning starts with selection of the proximal landing zone based on “healthy” aorta. The proximal landing zone should include at least a 2-cm length of “normal,” noncalcified, parallel aortic wall. The outer-to-outer aortic diameter should be more than 18 mm and less than 32 mm for pararenal aneurysms and more than 18 mm and less than 38 mm for TAAAs.6 Landing zone diameter should be no larger than the diameter of the next most proximal aortic segment.
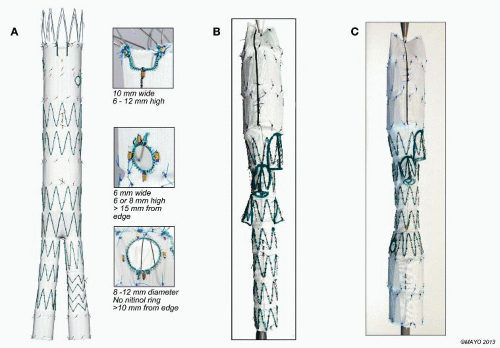
Fenestrated stent grafts are currently manufactured with three fenestration options: small and large circles and more proximal scallops (FIG 1A). Small fenestrations are 6 × 6 mm or 6 × 8 mm, created without crossing struts and reinforced by circumferential nitinol rings. Large fenestrations’ diameters are 8, 10, or 12 mm and may incorporate stent struts crossing the edge or middle of the circular defect, limiting space available for alignment stents. Scallops are contoured indentations along the upper edge of the main body endograft fabric, 10 mm wide and ranging in height from 6 to 12 mm, depending on individual patient anatomy.5
Device designs vary with aneurysm extent. For pararenal aneurysms, 70% of patients are adequately treated with two small fenestrations for the renal arteries and a scallop for the superior mesenteric artery (SMA).5 Suprarenal and type IV TAAAs typically require four fenestrations (no scallops). Extensive TAAAs (types I to III) need directional branches, particularly if the aortic diameter is relatively large or aneurysmal at the level of the visceral arteries. The combination of directional branches for celiac and SMA management with fenestrations for the renal arteries is increasingly popular.
These procedures require advanced endovascular skills and a comprehensive inventory of applicable catheters, balloons, and stents (Table 1). Dedicated training in fenestrated and branched techniques is highly recommended for physicians already experienced in endovascular disease management and ancillary procedures including renal and visceral artery disease management.
Patients with difficult aneurysm anatomy, chronic kidney disease, or advanced age are preadmitted for bowel preparation and intravenous hydration with bicarbonate infusion. Oral acetylcysteine is administered to minimize risk of periprocedural renal dysfunction following administration of iodinated contrast.
Hybrid, fixed imaging platforms are essential for optimal results of these complex procedures. Most are performed using general endotracheal anesthesia; local or regional anesthesia may be sufficient in select cases.
Intraoperative blood salvage systems (“cell saver”) are recommended for difficult cases and all TAAAs. The creation of large, impermeable pockets within dependent portions of the surgical drapes will facilitate pooling and collection via the cell saver.
The use of iodinated contrast is minimized by avoidance of power injector digital substraction angiography (DSA) runs during device implantation and side stent placement. Whenever possible, hand injections of dilute contrast (70% saline) are used to locate the side branches. Completion aortography is obtained only after all stents are positioned and postdilated, again using diluted contrast (50%).
To minimize contrast, precatheterization of targeted visceral arteries or use of onlay computed tomography (CT) images, when available, is recommended. In experienced hands, precatheterization adds little to the overall procedure time.
Table 1: List of Ancillary Tools Recommended for Physicians Performing Fenestrated Stent Graft Procedures | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Patients are positioned supine with the imaging unit oriented from the head of the table. Both arms are tucked for repair of pararenal aneurysms requiring up to three fenestrations.
Brachial artery access is used in patients treated by directional branches or those who need four fenestrations. The left arm is abducted and prepped in the surgical field up to the axilla. A working sterile side table is oriented in the same axis of the abducted arm for optimal support of necessary wires and catheters.
Electrocardiogram (EKG) leads, urinary catheter, and other monitoring cables and lines should be taped or secured so that they are not in the path of the x-ray beam of the fluoroscopic unit and do not impede movement of the C-arm gantry.
Access is established in the femoral arteries. Patients with small, calcified, or stenotic iliac arteries may require creation of an iliac conduit for safe device delivery.
Total percutaneous femoral access is the preferred approach in patients with noncalcified arteries or mild posterior plaque. The standard “preclose” technique enables complete hemostasis in more than 95% of patients irrespective of sheath diameter.7 When femoral arteries are small, calcified,
or bifurcate close to the inguinal ligament, standard surgical exposure and access is obtained. Proximal and distal control is obtained using vessel loops.
The left brachial artery is surgically exposed via small longitudinal incision in the upper arm, just proximal to the origin of the deep brachial artery.
Intravenous heparin (80 to 100 units/kg) is administered immediately after femoral and brachial access is established. An activated clotting time longer than 250 seconds is maintained throughout the procedure with frequent rechecks every 30 minutes. Prior to deployment of the stent graft, diuresis is induced with intravenous mannitol and/or furosemide.
Fenestrated-branched repair is currently performed using the Cook Zenith® stent graft lineage. Newer designs by Endologix (Ventana), Terumo (Anaconda), and Cook Medical (p-Branch) are under clinical investigation.
The Cook Zenith® fenestrated stent graft consists of a proximal fenestrated tubular component, a distal bifurcated universal component, and a contralateral iliac limb extension (FIG 1A). The fenestrated tubular component is custommade to fit the patient’s anatomy. Four to 6 weeks are required for manufacturing and delivery in the United States.
Bilateral percutaneous femoral access is established under ultrasound guidance; each femoral puncture is preclosed using two Perclose devices. Bilateral 8-Fr sheaths are introduced to the external iliac arteries over Benson guidewires (Cook Medical, Bloomington, IN). The guidewires are exchanged to 0.035-in soft glidewires and Kumpe catheters, which are advanced to the ascending aorta and exchanged for stiff 0.035-in Lunderquist guidewires (Cook Medical, Bloomington, IN).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree