Bone Marrow Examination and Techniques
Kaaren K. Reichard, MD
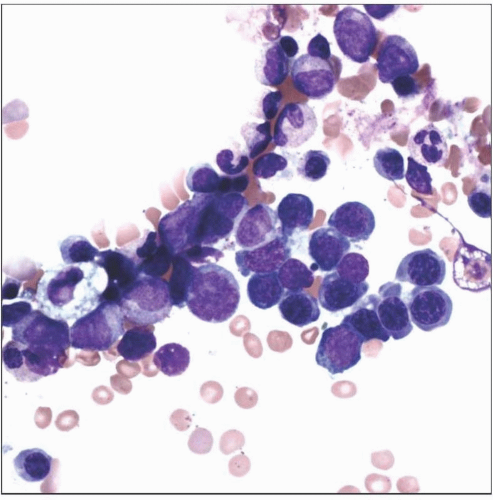 One important component of a thorough BME is a spicular, well-spread, and well-stained aspirate smear. Here one sees progressive maturation of the erythroid and granulocytic lineages. |
TERMINOLOGY
Abbreviations
Peripheral blood (PB)
Bone marrow (BM)
Bone marrow examination (BME)
Flow cytometry (FC)
Immunohistochemistry (IHC)
Fluorescence in situ hybridization (FISH)
Conventional cytogenetics (CC)
MICROSCOPIC FINDINGS
Morphology
Interpretation
Proper acquisition, preparation, and staining of BME components
See overview chapter on hematopoiesis for in-depth morphology review
Certain components of BME are optimal for certain tests (e.g., morphologic review, special studies)
PB: Dysplasia, increased blasts, abnormal lymphocytes, cytochemistry, FC, CC, FISH
BM aspirate: Assess trilineage hematopoiesis, dysplasia, increased blasts, lymphoid cells, histiocytic proliferations, cytochemistry, FC, CC, FISH
BM biopsy: Bone, stroma, cell composition and architecture, lymphoid aggregates, granulomas, IHC, molecular studies
Core biopsy can be disaggregated for FC, CC, FISH if BM inaspirable
Perform ancillary testing as indicated based on PB/BM review or protocol requirements
Based on review of PB and BM and clinical scenario or protocol requirements
Perform step sectioning to ensure adequate review of BM core biopsy
Particularly when assessing for involvement by focal/metastatic lesion
CLINICAL ISSUES
Indications
Investigation of unexplained PB abnormalities
Diagnosis of suspected primary hematopoietic neoplasm (e.g., leukemia, myeloproliferative neoplasm, myelodysplasia, myeloma)
Infectious disease work-up if other systemic investigations noncontributory
Evaluation of suspected constitutional disorder
Assess for storage disorder
Assess for involvement by metastatic neoplasm
Staging of lymphoma
Investigate radiologic bone or BM abnormality
Ongoing monitoring after therapy
After BM transplant to assess for BM recovery
Protocol requirement
Contraindications
Severe coagulopathy
Severe bleeding disorder
Overlying skin/soft tissue infection
Complications of BM Biopsy
Rare but well recognized
Incidence not rigorously documented
In UK survey, 0.07% incidence of adverse events after BM aspiration &/or biopsy
Hemorrhage most common
Other: Ongoing pain, fracture, anaphylaxis, and infection
Prebiopsy Preparations
Discussions regarding
Indications for procedure
Potential complications
Steps in procedure
Duration of procedure
Postprocedure expected discomfort and instructions
Time interval for test results
Appropriate materials should be available as needed (e.g., media for ancillary studies)
Ample slides for touch preparation and bone marrow aspirate smears
Sodium heparin-coated syringes for cytogenetics, flow cytometry
TECHNICAL ISSUES
Sites of BME
Posterior iliac crest
Standard site
Unilateral specimen adequate for work-up of most conditions
Bilateral specimens uncommon
Used for staging of BM involvement in past
Anterior iliac crest
Rare
Used when posterior iliac crest is not an option
Sternum
Exceedingly rare; high risk of sternal plate puncture
Aspiration specimen only; guard required on needle
Components of BME
CBC data
Peripheral blood smear
BM aspirate
BM touch preparation
BM clot section
BM trephine biopsy
Adequacy of BM Aspiration and Biopsy
BM aspiration
Obtain at least 3 particles per slide
Obtain at least 4 slides
2 for routine staining
1 for iron (as needed)
1 for potential ancillary tests (e.g., cytochemistry, FISH)
Acquire additional material for ancillary tests (as needed)
Optimal Wright (or equivalent) stain for satisfactory cytologic assessment
Heparin-coated syringe for FC, CC, molecular genetic studies
BM trephine biopsy
1.5-3 cm in length
No significant aspiration artifact
Perform biopsy prior to aspiration
Perform touch preparation (see BM touch preparation section)
Particularly if BM aspirate is suboptimal
Ensure adequate and proper decalcification, formalin fixation and processing, sectioning, and staining
Lengthy decalcification results in
Suboptimal morphology
Suboptimal immunohistochemistry
Suboptimal special stains (reticulin)
BM touch preparation
Adequate stain for cytologic assessment
Particularly in event of no/suboptimal aspirate
Important for potential ancillary techniques (e.g., cytochemistry, FISH, iron stains)
Particularly if BM aspirate is hemodilute secondary to tumor infiltration, fibrosis
≥ 3 air-dried imprints
REPORTING CRITERIA
Individual BM Reports
Should be completed on average within 3 days
Integrate additional ancillary testing information
IHC
FC
Cytochemistry
Utilize standardized template &/or synoptic reporting
Organize report such that key diagnostic and prognostic information is readily available to clinician
Final Integrated BM Reports
Should be promptly completed upon finalization of last piece of testing data (e.g., CC, FISH, &/or molecular studies)
Report significance of ancillary studies
Report prognostic implications
Suggest any additional testing as needed
Utilize standard classification systems if possible
Synoptic (or similar) reporting allows for standardization and reproducible location of key information
ANCILLARY TECHNIQUES
Cytochemistry
Myeloperoxidase (MPO) and nonspecific esterase (NSE) (a.k.a. α-naphthyl butyrate esterase) are most common
Requires unfixed/unstained specimen
PB
BM aspirate/touch preparation
Cytospin preparation from FC/CC aspirate
MPO
Stains granules within neutrophilic lineage
Intensity of staining greatly increases at promyelocyte stage of maturation
Blasts of myeloid lineage show scattered positive granules
May rarely see sparse positive granules in blasts of monocytic &/or lymphoid lineage
All normal myeloblasts should exhibit scattered MPO positivity
MPO positivity does not discriminate neoplastic from nonneoplastic blasts
Not all myeloblasts are MPO positive
Need immunophenotyping and genetic studies
Fast
Technique usually takes ˜10 minutes to perform
NSE
Stains cells within monocytic lineage
Monoblasts
Promonocytes
Monocytes and histiocytes
Diffuse cytoplasmic brown staining
Assay is technically tricky
Need reliable external &/or internal positive control
Positivity does not discriminate neoplastic from nonneoplastic monocytes
Requires morphologic integration
Special Stains for Iron
Iron studies
Nonenzymatic stain; Prussian blue
Highlights iron particles/stores as blue-green cytoplasmic granules &/or clumps
Assess 2 features
Erythroid iron
Macrophage stores
Erythroid iron
Need adequate, unstained, air-dried BM aspirate smear/touch preparation
Normal red cell iron
˜ 20-50% of red blood cell precursors demonstrate 1-3 small cytoplasmic granules
Pathologic red cell iron
Abnormal size (large), shape (chunky), or location (ring) of granules
Ring sideroblast is defined by World Health Organization as 1/3 of red blood cell nucleus tightly surrounded by 5 or more iron granules
Storage iron
Grading may be semiquantitatively assessed (normal, none, increased, decreased) or assessed by grading scale (0-6)
Need good-quality BM aspirate with sufficient particles
Need adequate positive control to insure accuracy
May have heterogeneous iron deposition in BM aspirate
Formalin fixation may interfere with staining of iron stores (ferritin)
Special Stains for Fibrosis
2 types of BM fibrosis: Reticulin and collagen
Reticulin fibrosis
May be seen in various neoplastic and nonneoplastic disorders
Detected by silver stain
Reticulin is present normally in bone marrow
Seen as scant thin fibers around small vessels and rare sinusoids
Often reversible with eradication of underlying pathology
Variability occurs within staining; ensure reproducibility
Preanalytical factors may affect quality: Fixation, decalcification, thick sections, manual vs. automated procedure
Collagen fibrosis
Detected with trichrome stain
Not normally present in bone marrow
Unlikely to be reversible
Grading scale
Recent proposal of 0-3 scale
Immunohistochemistry
Performed on fixed specimen (BM clot or core biopsy)
Numerous IHC stains available
Lymphoid cells
B; CD19, CD20, CD79a, CD22
T; CD1a, CD2, CD3, CD4, CD5, CD7, CD8
NK; CD2(+), sCD3(-), CD16, CD56
Cells in neutrophil lineage
CD13, CD33 (not routinely available by IHC), MPO
Monocytic cells
CD163, CD68, CD4 (weak)
Blasts (not lineage-specific)
CD34, TdT, CD117
Erythroid cells
CD117 (weak +) in pronormoblasts, hemoglobin A, glycophorin A, CD71
Megakaryocytic lineage
CD31, CD41, CD42b, CD61
Mast cells
CD117, tryptase
Plasma cells
CD138, CD38, cytoplasmic κ and λ
Indications for IHC assessment of BM biopsy specimens
Evaluate for morphologically occult process (use CD3, CD20, CD34)
Evaluation of unexplained hypocellular BM (e.g., hairy cell leukemia, increased blasts)
Evaluation of unexplained hypercellular marrow (e.g., increased blasts, increase in mast cells)
Evaluation of lymphoid aggregates if suspicious (e.g., paratrabecular aggregates) &/or flow suboptimal or negative
Caveats of IHC
Variable IHC results depending on fixation, decalcification, antibody clones/dilution
Comparative features of IHC vs. FC
Slower turnaround time (TAT) for IHC
Generally uniparametric; occasionally dual parametric IHC
Preserved architecture in tissue section
No significant risk of loss of antigens if properly preserved (IHC)
Assess infectious agents, proliferation rate, aberrant oncoproteins
e.g., EBER, Ki-67, p53, cyclin D1, ALK1
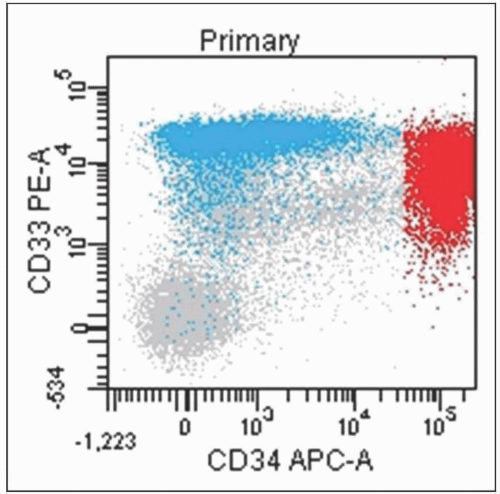
Flow Cytometry
Performed on variety of fresh specimens
Peripheral blood
Bone marrow aspirate
Fine needle aspirations
Disaggregated bone marrow core biopsy
Antibodies overlap with IHC; however, some key differences
CD13/CD33 readily available by FC (myeloid)
CD14/CD36/CD64 (monocytic)
Surface κ and λ (mature B cells)
T-cell receptor Vβ subsets
Indications for FC in BME
Determine lineage of new acute or chronic leukemia
Determine lineage of new lymphoma
Subclassify new leukemia or lymphoma (as possible)
Assess for increased blasts
Readily detect aberrant antigen expression
Identify abnormal plasma cell population
Minimal residual disease testing
Advantages of FC in BME
Fast
Multiparametric
Increased antigen sensitivity
Disadvantages of FC in BME
Requires fresh tissue
Results limited to accuracy of specimen representation (e.g., hemodilution, cells of interest absent, poor viability)
Caveats of FC