Bone Lesion/Tumor: Diagnosis and Margins
SURGICAL/CLINICAL CONSIDERATIONS
Goal of Consultation
Determine if a bone lesion is benign/reactive or malignant
Evaluate margins after a definitive resection of a malignant bone tumor
Change in Patient Management
Reactive lesions may be followed clinically or excised
Benign neoplasms are often excised or curetted without need to document negative margins
Low-grade malignancies are resected, often widely (if possible) and with margin assessment
Complete curettage may also be attempted in some well-localized tumors
High-grade malignancies may be treated with radiation/chemotherapy prior to resection or resected before treatment
Positive bony &/or marrow margins may generate additional resection or possibly closure and subsequent adjuvant therapy
Clinical Setting
Patient age ranges from pediatric to adult
Lytic &/or blastic lesions identified on radiograph
May be incidentally discovered on plain film during evaluation for other conditions
Bone destruction &/or soft tissue invasion often apparent in locally aggressive &/or malignant tumors
Malignancies are more likely than benign lesions to cause pain
SPECIMEN EVALUATION
Radiograph
Review of appropriate patient radiographs prior to receipt of specimen is highly recommended
Radiologist’s differential diagnosis is essential for correlation with pathologic findings
Knowing the specific bone as well as anatomic region of bone involved is diagnostically useful information
Identification of an aggressive growth pattern radiographically can be essential in helping classify a low-grade bone malignancy intraoperatively
Gross
Bone specimens sent for intraoperative consultation and diagnosis are almost invariably composed of small fragments of soft tissue and bone
Intraoperative margins sent for bone tumors are either peripheral soft tissue margins or intramedullary marrow margins
Frozen Section
Hard bony fragments should be separated from soft tissue fragments
Softer bony fragments can be frozen, but harder bone may damage cryostat blade &/or distort frozen section
Tissue that can be cut easily with a scalpel blade can generally be sectioned on a cryostat
All tissue should be frozen unless entire lesion has been curetted and sent to pathology
If completely curetted, representative tissue from specimen usually suffices
These specimens are usually from presumptively benign lesions
Cytology
Touch prep slides of lesional tissue may be helpful if metastatic carcinoma is suspected
MOST COMMON DIAGNOSES
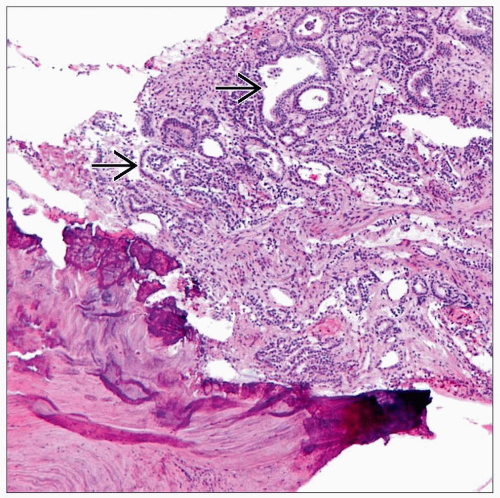
Metastatic Cancer
Far more common than primary bone tumors
Carcinoma is most common, but melanoma, sarcoma, or lymphoma may also metastasize to bone
Must always be considered in patients > 45 years of age
Patients often have history of cancer elsewhere as well as prior metastatic disease (e.g., to lymph nodes, lung)
Any bone may be involved
Most often proximal humerus, proximal femur, or spine
Histologic appearance depends on primary tumor
Usually adenocarcinoma (most common primary sites include breast, lung, kidney, prostate, liver, and thyroid)
Some metastatic carcinomas (particularly from prostate) are osteoblastic and produce focal to abundant woven bone
Immunohistochemical studies performed on permanent sections may be necessary for classification if no primary site is known
Decalcification can diminish immunoreactivity for some antigens
If possible, softer areas of tumor should be separated and not decalcified
Osteosarcoma
Most commonly seen in young patients between 10 and 20 years of age
2nd peak occurs in patients > 50 years of age who have a predisposing condition (e.g., radiation, Paget disease)
Imaging often shows a large permeative and destructive lesion in metaphysis/diaphysis of a long bone, most commonly around region of knee (˜ 50% of cases)
Most cases show sheets of frankly malignant cells with a high level of mitotic activity
Diagnosis depends upon identification of pink seams of osteoid or immature bone directly produced by malignant cells
Some tumors may show focal or prominent cartilaginous differentiation, leading to possible confusion with chondrosarcoma
Younger age and presence of cellular sheets of malignant cells (± osteoid) favor osteosarcoma
Other variant morphologies include fibroblastic (spindled), small cell, clear cell, telangiectatic, and giant cell rich
Enchondroma
Often painless unless associated with a fracture
Imaging often shows a small, well-circumscribed lesion without locally aggressive features in diaphysis/metaphysis of long bones
In long bones: Generally composed of fragments and nodules of bland hyaline cartilage, often with a thin pink seam of peripheral bone
Chondrocyte cellularity is often low and with minimal or no nuclear atypia
In small bones of hands and feet: Often much more cellular and may demonstrate mild nuclear atypia and myxoid matrix degeneration
Clinical and radiologic correlation to evaluate for pathologic cortical bone disruption and soft tissue extension is most helpful in excluding a chondrosarcoma
In some cases, it can be extremely difficult, if not impossible, to histologically distinguish an enchondroma from a low-grade chondrosarcoma
Correlation with imaging is often critical in making this distinction
In ambiguous cases, a diagnosis of “low-grade cartilaginous lesion, final diagnosis deferred to permanent sections” is appropriate
Any subsequent curetted tissue should be submitted for permanent histologic evaluation
Chondrosarcoma
Often painful
Imaging generally shows an irregular, large, and destructive tumor, often with cortical disruption and soft tissue extension, involving metaphysis/diaphysis of long bones
Clear cell chondrosarcoma (variant) characteristically arises in epiphysis of long bones
Cellularity and chondrocyte nuclear atypia varies widely depending on grade, but most cases show a higher degree of both than seen in benign cartilage or enchondroma
Chondrocyte necrosis and prominent matrix degeneration are also more common in chondrosarcoma
Chondrosarcoma of small bones of hands and feet is very rare and requires evidence of true bony or soft tissue invasion for diagnosis
Dedifferentiated chondrosarcoma may show cellular, noncartilaginous high-grade sarcoma or neoplastic cartilage or both, depending on what portion of tumor is sampled at time of frozen section
Ewing Sarcoma
Most common in patients < 20 years of age
Painful, enlarging mass, often with soft tissue swelling
Imaging shows a large, poorly defined destructive tumor most often centered in metaphysis or diaphysis of long bones or flat bones of pelvis
Sheets of monomorphic small, round cells with scanty cytoplasm (“small round blue cell tumor”) within a variably fibrous background
Most cells have finely distributed chromatin and inconspicuous nucleoli
Necrosis is often abundant
Diagnosis of “malignant small round blue cell tumor, final diagnosis deferred to permanent sections” is often appropriate
Must exclude other morphologically similar neoplasms such as small cell osteosarcoma, mesenchymal chondrosarcoma, or lymphoma
Confirmatory ancillary testing (IHC, FISH, etc.) is required on permanent sections
Giant Cell Tumor
Typically occur in adults (age range: 25-45)
Localization to epiphysis of a long bone is characteristic, similar to chondroblastoma and clear cell chondrosarcoma
Also shows metaphyseal extension in many cases
Tumor is often locally aggressive and may show limited soft tissue extension
Composed of sheets of multinucleated osteoclast-like giant cells in a background of bland, monomorphic ovoid or spindled stromal cells
Giant cells may be very large (> 50 nuclei)
Stromal cells should not show malignant cytologic features or atypical mitoses
Evidence of frank malignancy should raise possibility of other tumors, such as metastatic carcinoma, osteosarcoma, dedifferentiated chondrosarcoma, etc.
May contain areas of aneurysmal bone cyst (ABC)
Epiphyseal location suggests secondary ABC over primary ABC
Chondroblastoma
Typically occurs in skeletally immature patients, with open growth plates (age range: 10-25 years)
Localization to epiphysis or apophysis of a long bone is characteristic
Imaging shows well-defined tumor of variable size, often with sclerotic margins
Contains large number of osteoclast-like giant cells, similar to giant cell tumor
Predominant cell type (chondroblast) is generally pink, epithelioid, and contains a nucleus that is sometimes grooved
Identification of stromal “chicken wire” calcification and fragments of cartilage is common
Distinguishing chondroblastoma from giant cell tumor
Young age, epithelioid “fried egg” cells with grooved nuclei, “chicken wire” calcification, and presence of cartilage all favor chondroblastoma
May contain areas of aneurysmal bone cyst
Fibrous Dysplasia
Most common in young to middle-aged patients; often asymptomatic
May present as solitary or multiple lesions (either within same or different bones)
Most common in long bones
Other sites (e.g., ribs and craniofacial bones) may be involved
Imaging shows a well- circumscribed intramedullary tumor, most commonly in diaphysis and metadiaphysis
Composed of a mixture of fibroblasts and immature (woven) bone trabeculae
Spindled fibroblasts are bland and may demonstrate a storiform growth pattern
Stroma is often collagenous but may be myxoid
Woven bone trabeculae are characteristically irregularly shaped, discontinuous, and often lack osteoblastic rimming
Woven bone production can be focal in some tumors and may not be sampled, potentially leading to diagnostic difficulty
Does not demonstrate an infiltrative or permeative growth pattern through preexisting lamellar bone (finding suggests low-grade osteosarcoma)
Aneurysmal Bone Cyst (ABC)
Most commonly arise in young patients (< 20 years) in metaphyseal region of long bones
Imaging generally shows an expansile, lytic, and cystic lesion, often with fluid levels due to blood
Composed of variably thick cyst walls containing bland spindled to ovoid cells, scattered giant cells, and focal seams of osteoid
Frankly malignant cells are inconsistent with diagnosis and may suggest telangiectatic osteosarcoma
Epiphyseal lesions with the appearance of ABC are usually different tumors (giant cell tumor, chondroblastoma, etc.) with a secondary ABC
Osteomyelitis
Patients often complain of pain and systemic symptoms
Can easily mimic a neoplasm on imaging
Early osteomyelitis shows fragments of mature lamellar bone associated with granulation tissue, necrotic bone, and collections of acute inflammatory cells
Chronic osteomyelitis often shows intertrabecular loose fibrosis and a more prominent plasma cell infiltrate
Fibrous dysplasia and low-grade osteosarcoma show a cellular spindle cell proliferation rather than loose fibrosis
Plasma cell neoplasms contain sheets of plasma cells and lack granulation tissue
Langerhans Cell Histiocytosis (Eosinophilic Granuloma)
Usually presents in first 3 decades of life
May be solitary (eosinophilic granuloma) or multifocal
Systemic disease involves multiple organ systems and frequently occurs in first 2 years of life
Most common in craniofacial bones but can involve any bone
Imaging usually shows small, well-defined lesion (may have “punched out” appearance)
Composed of irregular sheets and nests of pink histiocytic cells (Langerhans cells) within a typically prominent mixed chronic inflammatory infiltrate
Langerhans cell nuclei are generally ovoid with nuclear grooves (coffee bean shaped) or notched
Eosinophils are variably prominent, and may show eosinophilic abscess formation
Reactive germinal centers may be present
Inflammation may obscure diagnostic Langerhans cells
Metaphyseal Fibrous Defect (Nonossifying Fibroma/Fibrous Cortical Defect)
Most common in 2nd decade of life
Imaging shows small, well-demarcated, cortical-based lesion in metaphysis of long bone
Painless and asymptomatic, unless large or associated with fracture
Composed of bland spindled fibroblasts in a storiform growth pattern associated with scattered multinucleated osteoclast-like giant cells
Number of giant cells may be striking, but sheets of giant cells are not seen (as in giant cell tumor)
Xanthomatous change and chronic inflammation are commonly identified
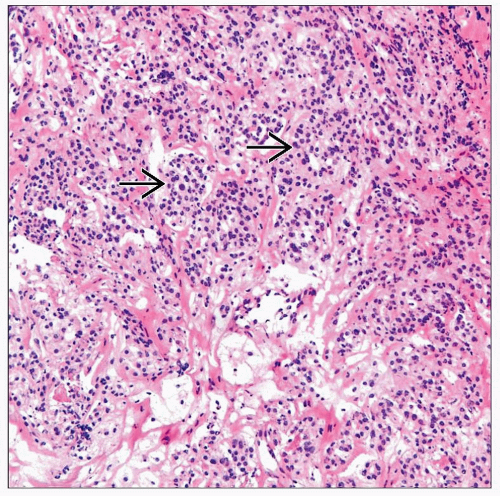
Plasma Cell Neoplasm
Very common primary malignant bone tumor
Most patients are middle aged or older
May present as a localized lesion (plasmacytoma) or as a component of widespread disease (plasma cell myeloma)
Composed of sheets of plasma cells demonstrating varying degrees of nuclear atypia
Poorly differentiated plasma cells may be difficult to distinguish from carcinoma, and clinical/radiographic correlation or even deferral may be required
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree