|
Papillary Urothelial Neoplasm of Low Malignant Potential (PUNLMP) |
Low-Grade Papillary Urothelial Carcinoma |
Age |
Mean age mid-60s with wide range. Can rarely be seen in children |
Mean age mid-60s with wide range. Can rarely be seen in children |
Location |
Anywhere with urothelial lining |
Anywhere with urothelial lining |
Symptoms |
Typically gross or microscopic hematuria |
Typically gross or microscopic hematuria |
Signs |
At cystoscopy, papillary lesion. May be solitary or multifocal |
At cystoscopy, papillary lesion, typically solitary or multifocal |
Etiology |
Thought to be the same as urothelial carcinoma |
Numerous environmental risk factors. Most common is history of smoking, accounting for 60% and 30% of all urothelial carcinomas in males and females, respectively. Other risk factors include exposure to various chemicals, heredity, infection, prior radiation, and prior chemotherapy with cyclophosphamide |
Histology |
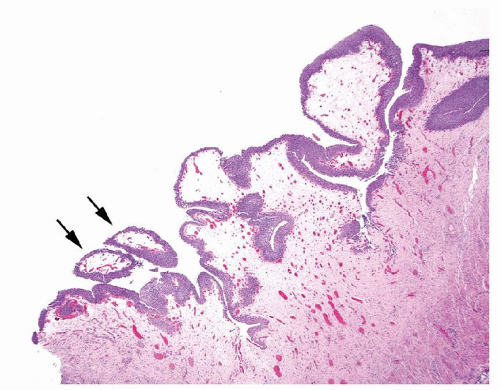
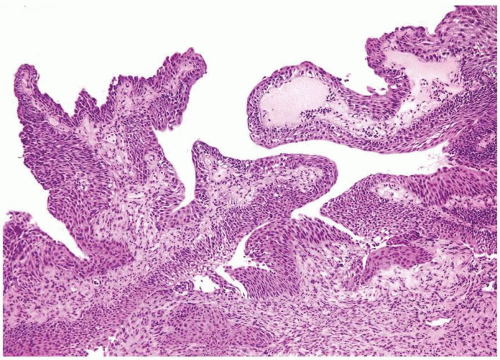
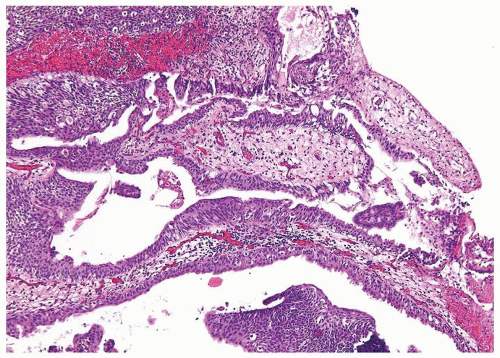
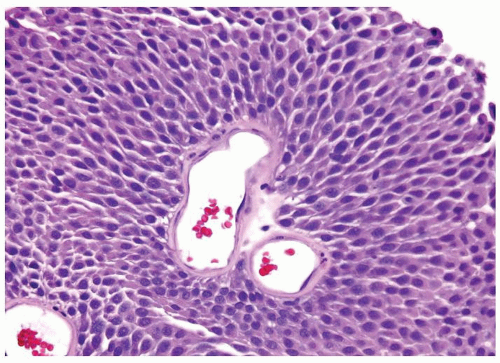
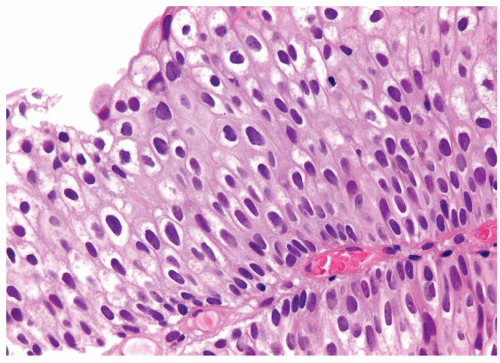
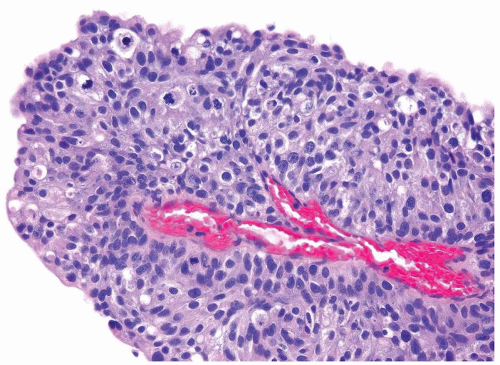
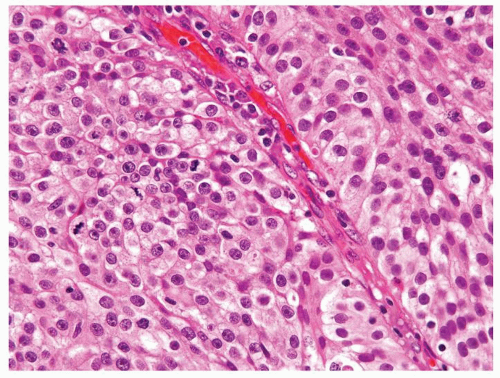
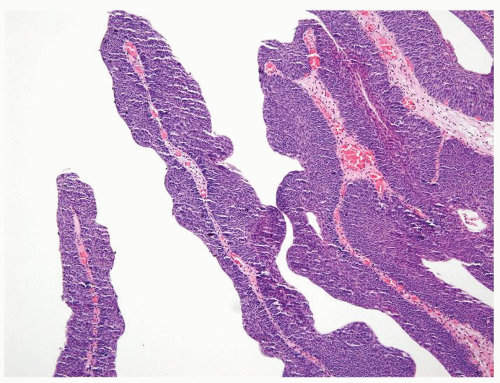
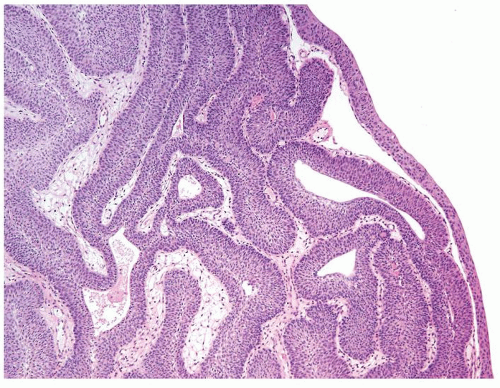
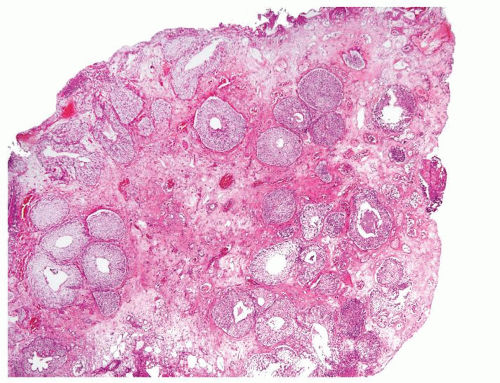
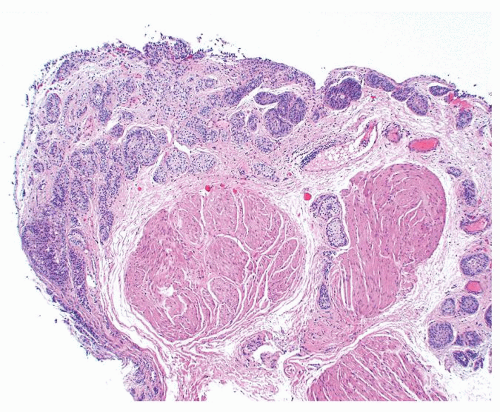
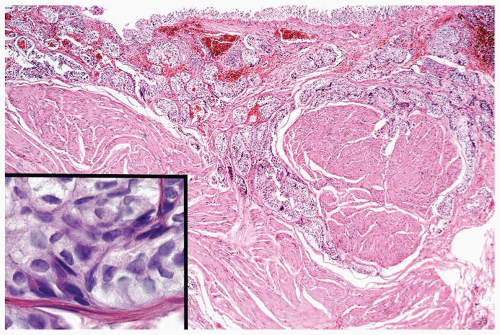
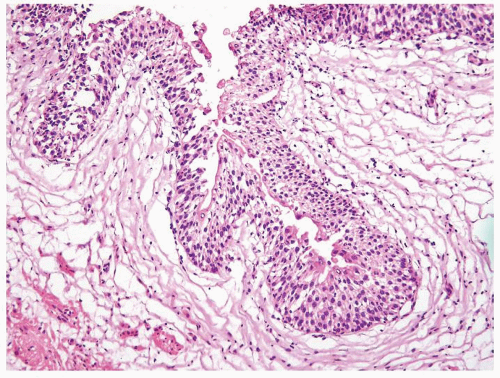
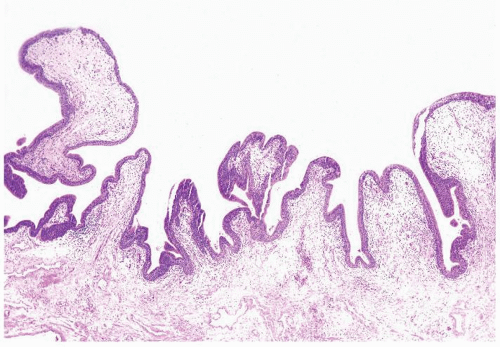
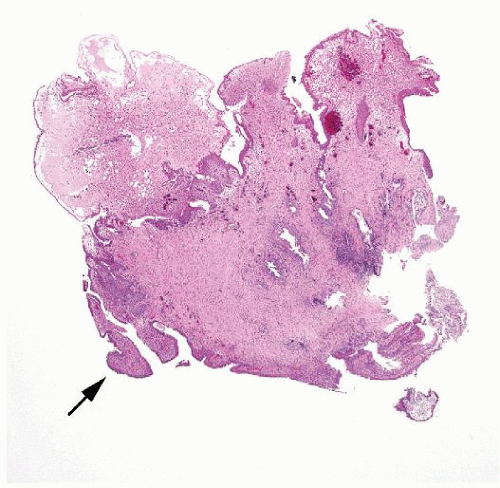
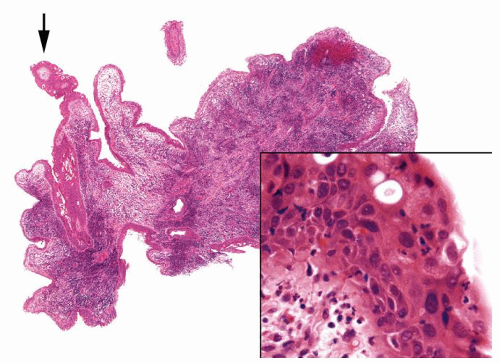
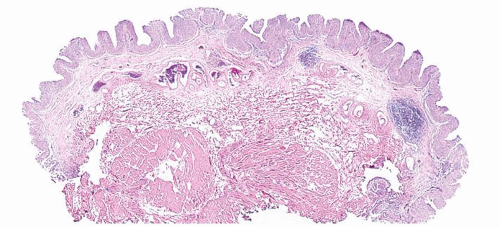
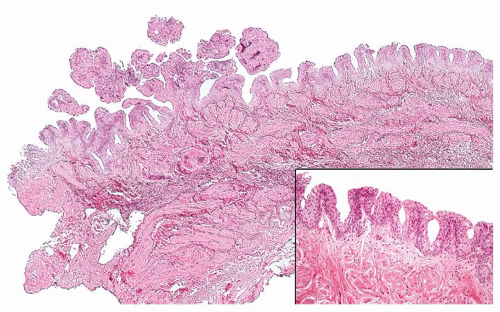
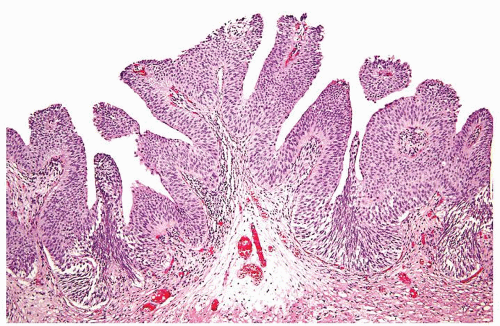
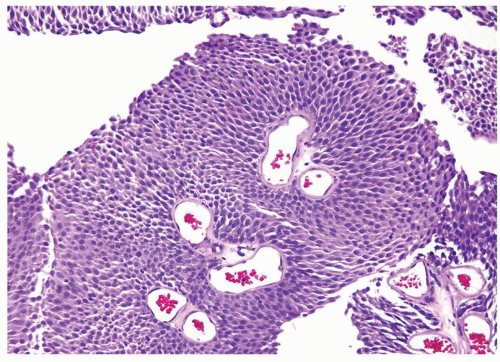
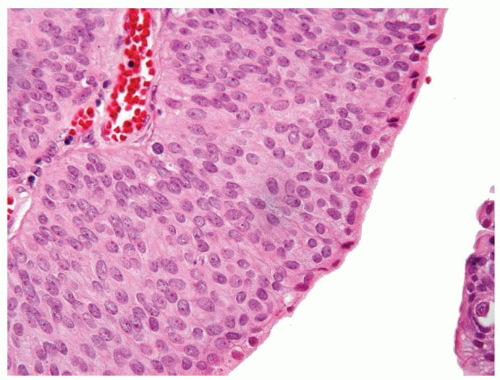
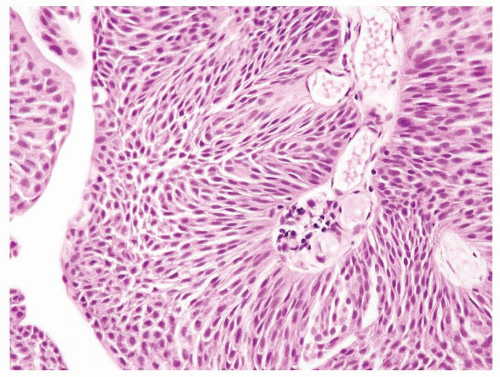
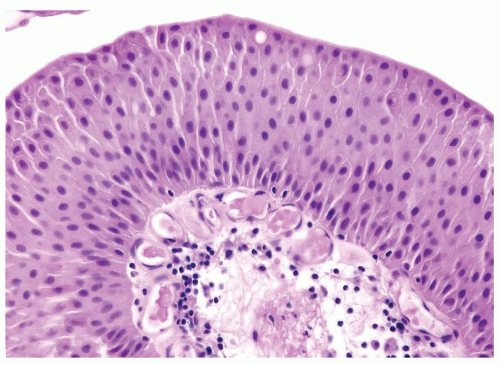
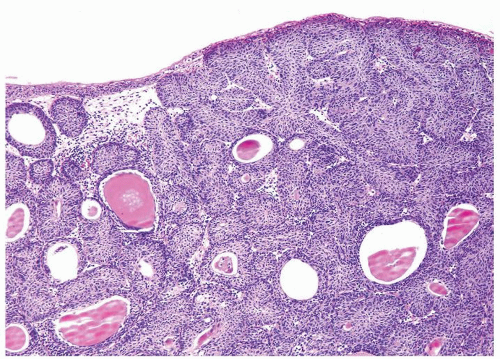
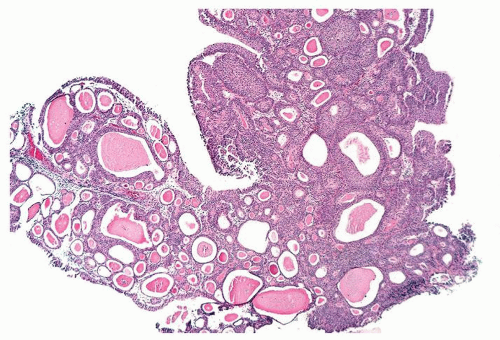
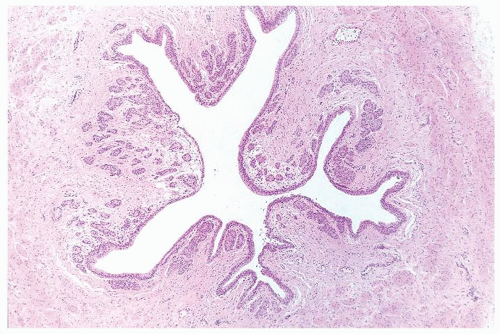
Ranges in size. Can be large requiring multiple slides to totally submit
Usually discrete, nonfused papillae but some may be fused
Often with a prominent inverted growth pattern
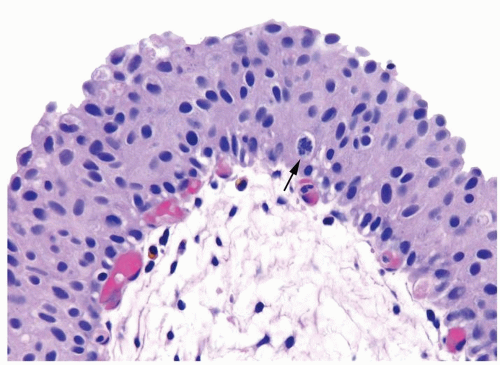
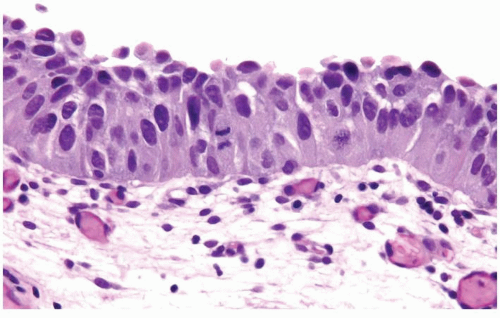
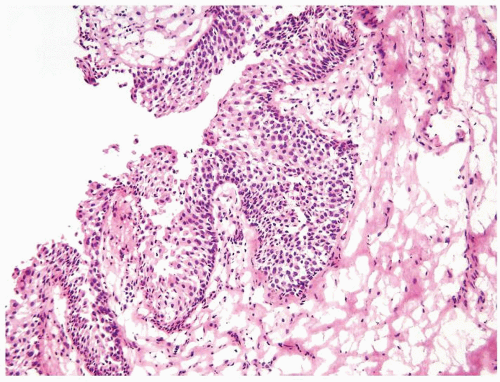
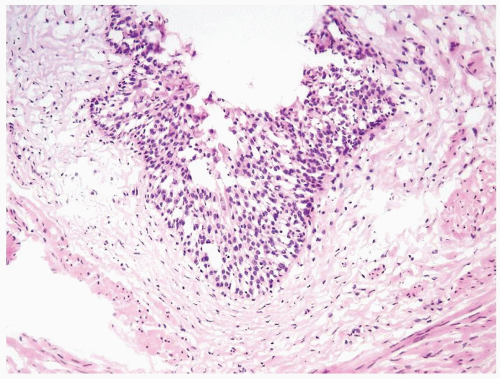
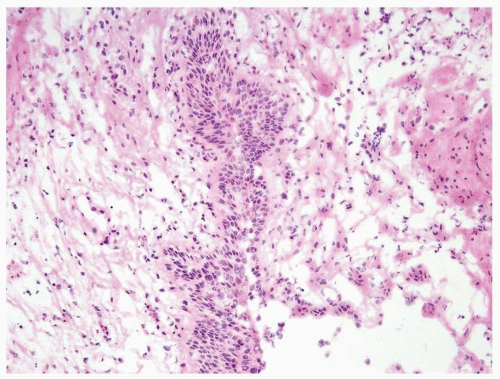
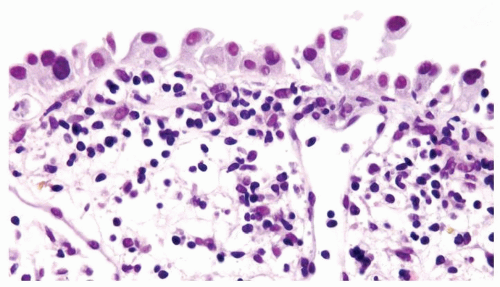
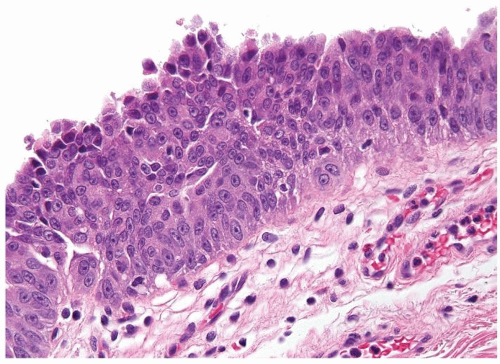
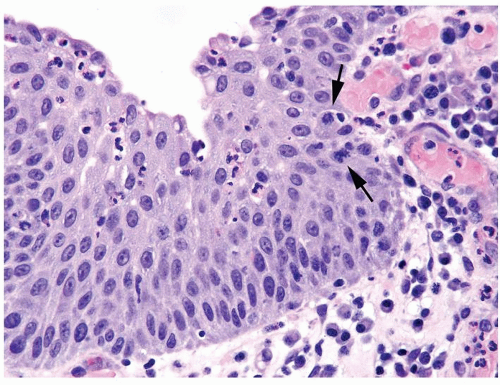
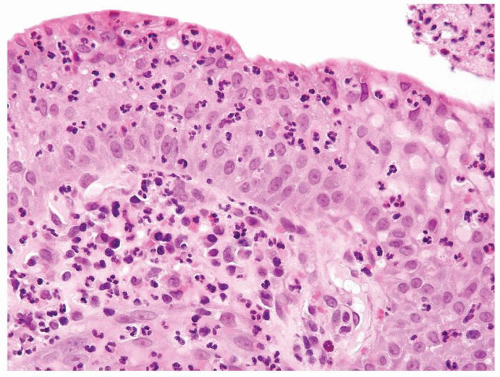
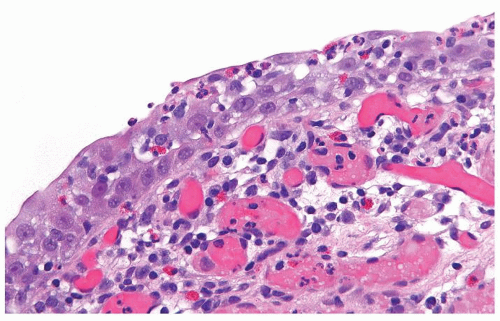
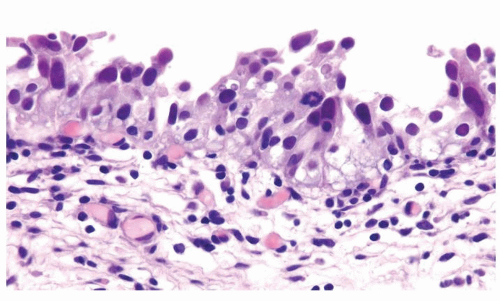
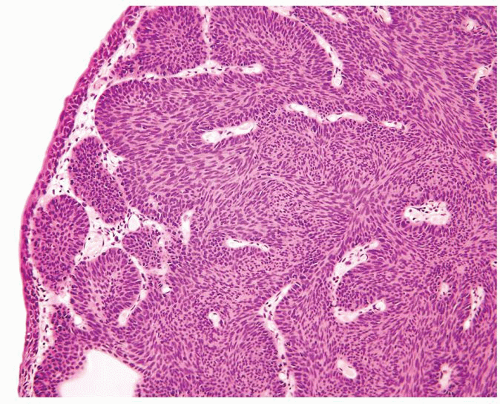
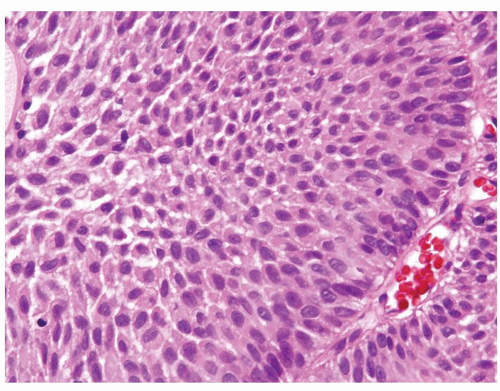
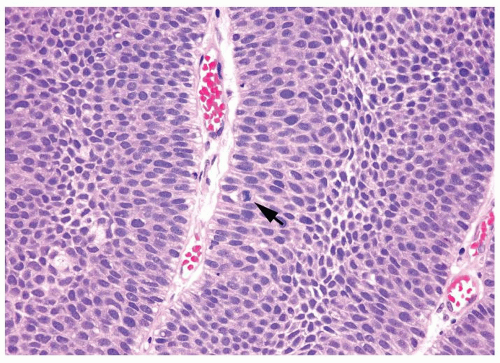
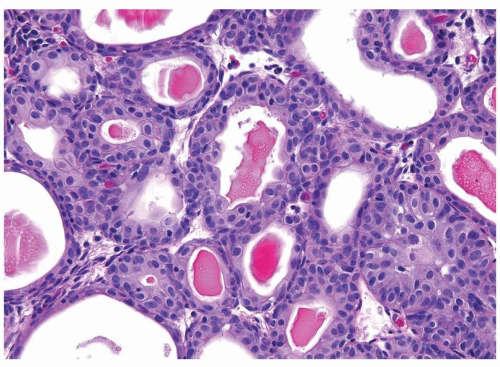
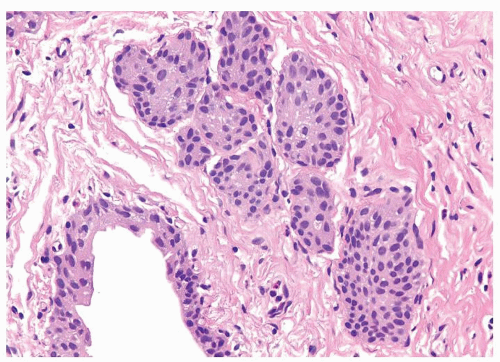
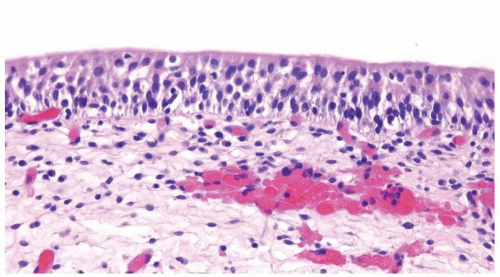
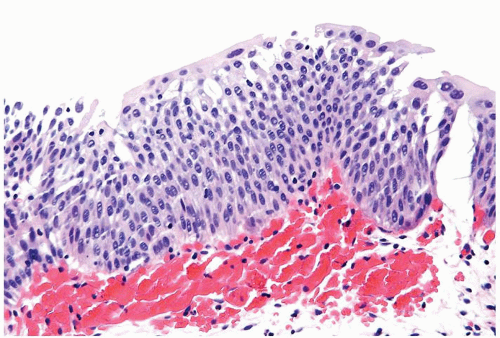
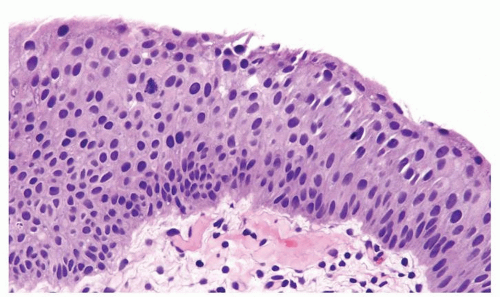
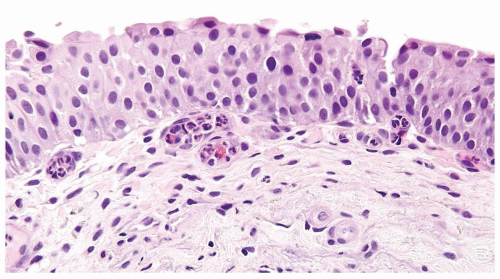
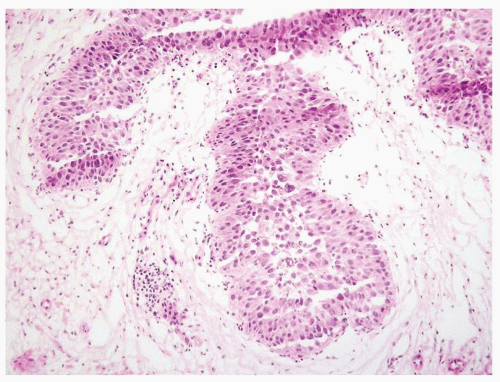
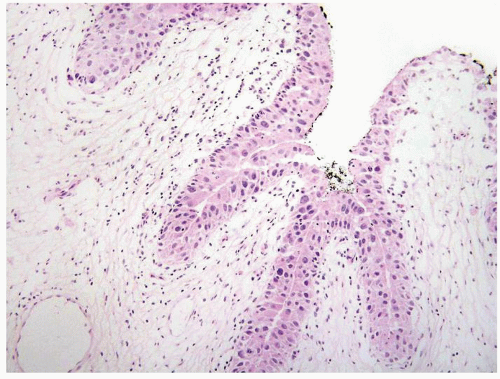
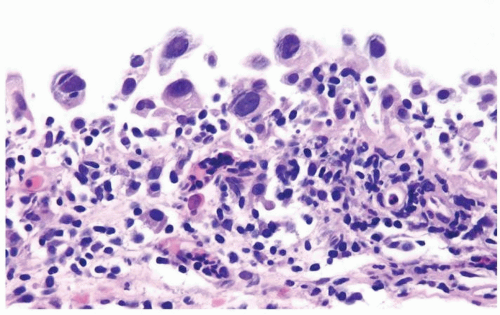
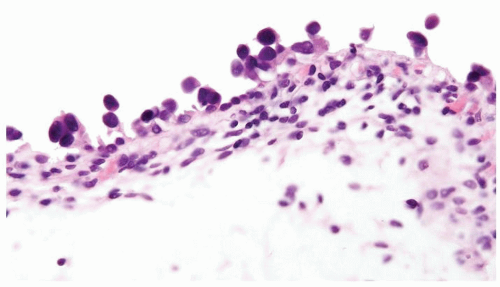
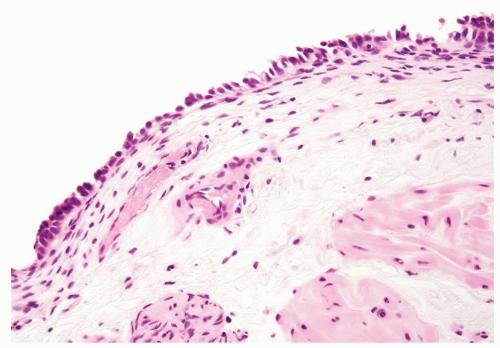
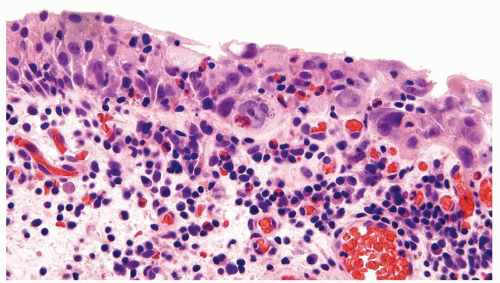
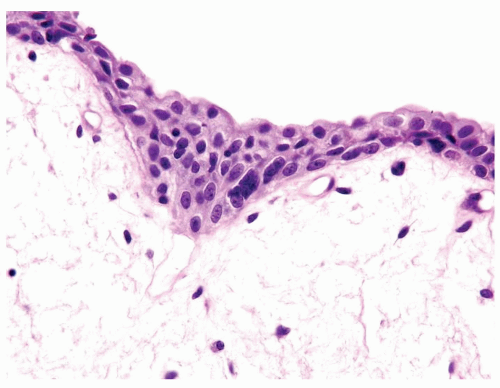
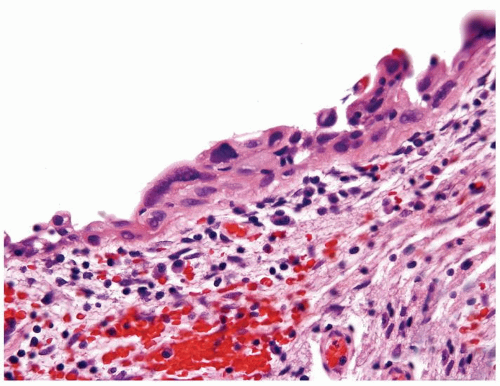
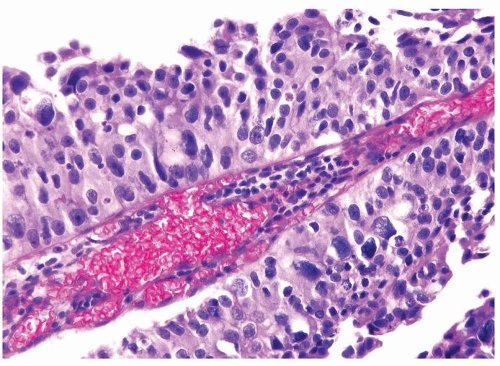
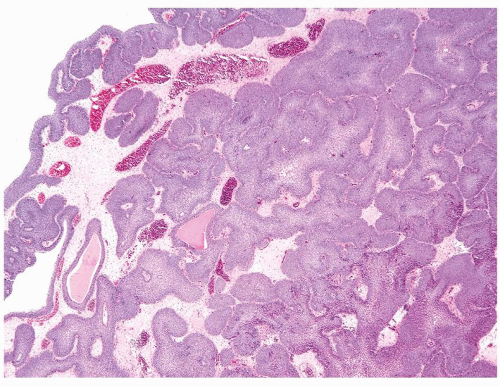
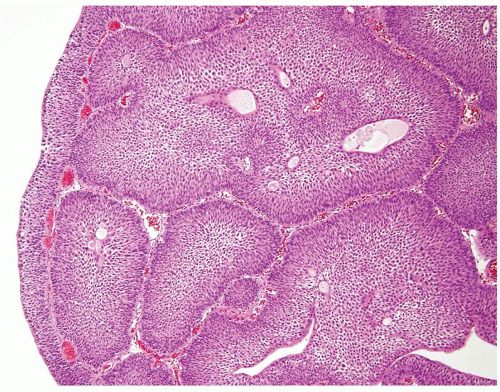
The urothelium is overtly thick, even at low magnification (Figs.3.10.1, 3.10.2, 3.10.3, 3.10.4)
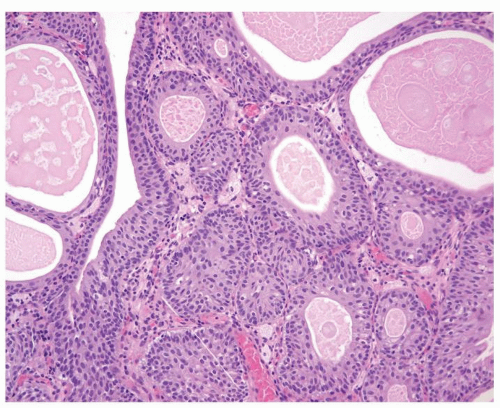
Polarity strictly maintained
Cytology is normal or at most slightly enlarged nuclei some with nuclear grooves (Figs.3.10.1, 3.10.2, 3.10.3, 3.10.4)
Every microscopic field is monotonous with uniformly sized and shaped nuclei having light chromatin
Nucleoli indistinct
Mitotic figures virtually absent or at most rarely seen toward the basement membrane
|
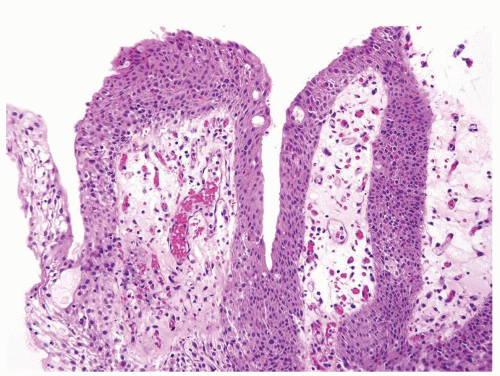
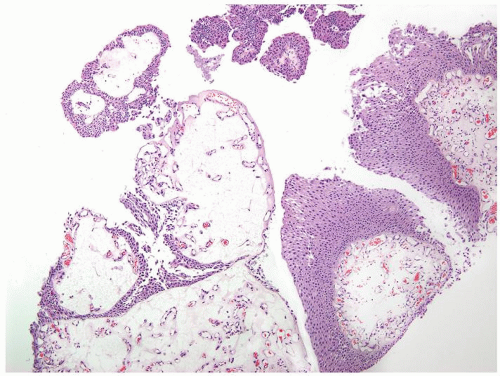
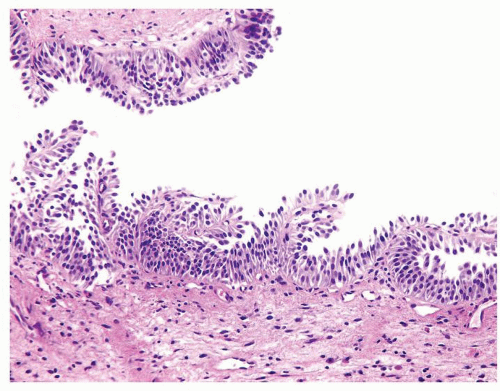
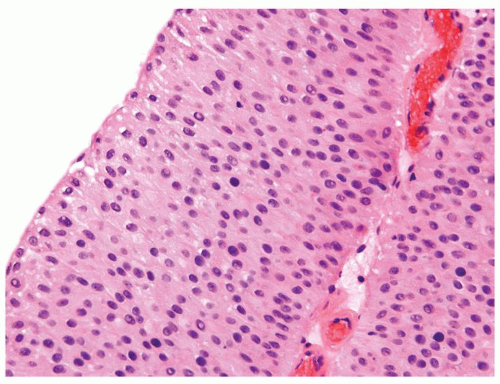
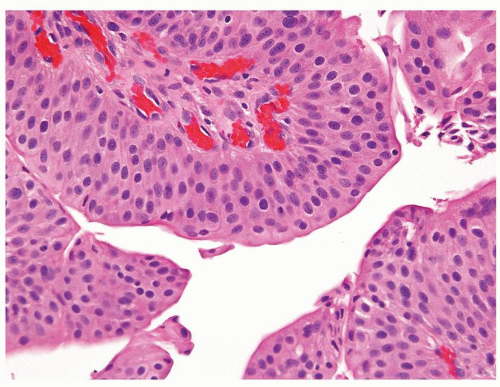
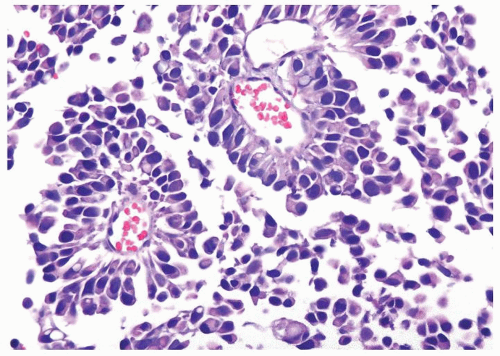
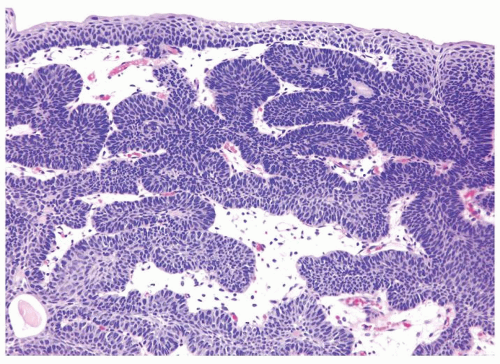
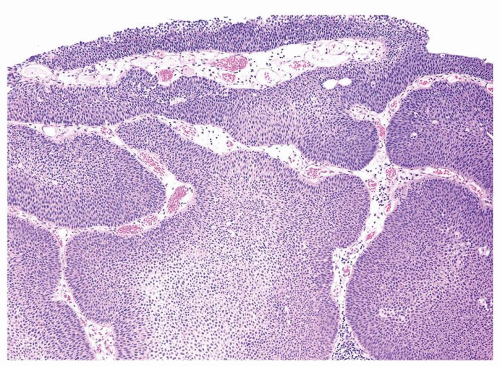
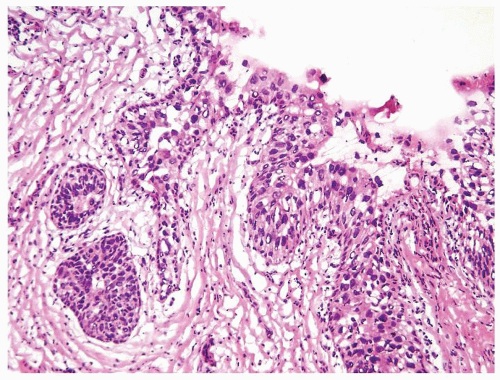
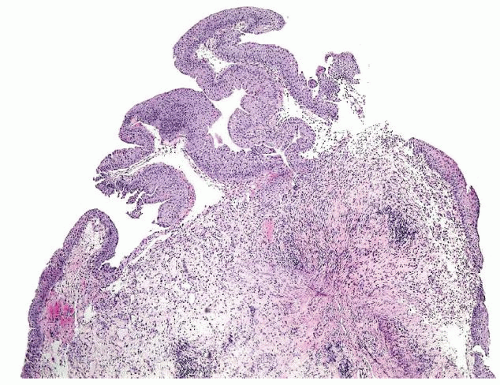
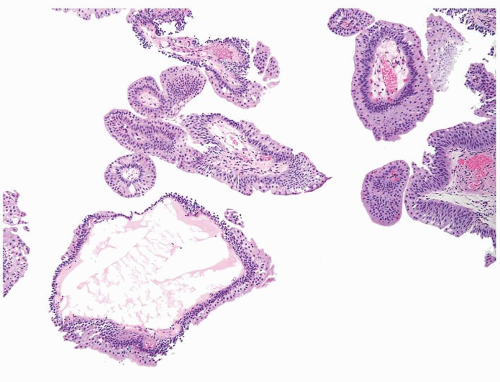
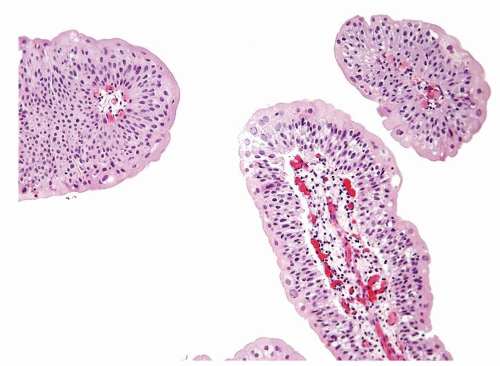
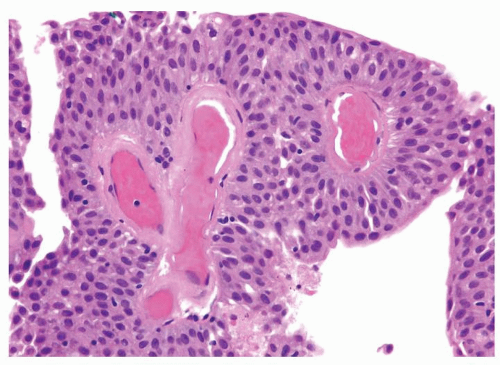
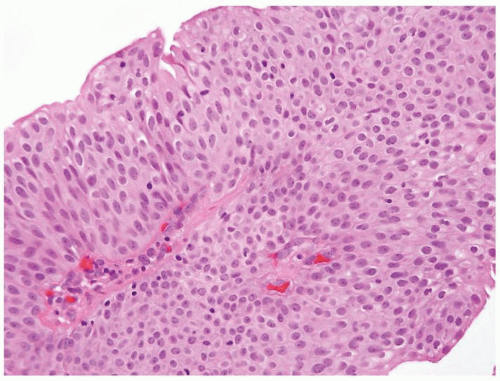
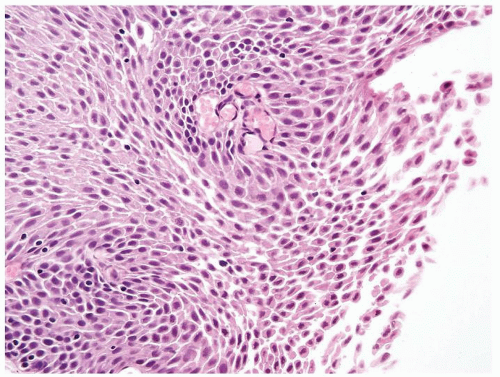
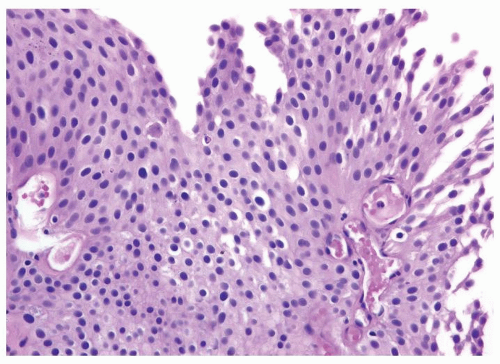
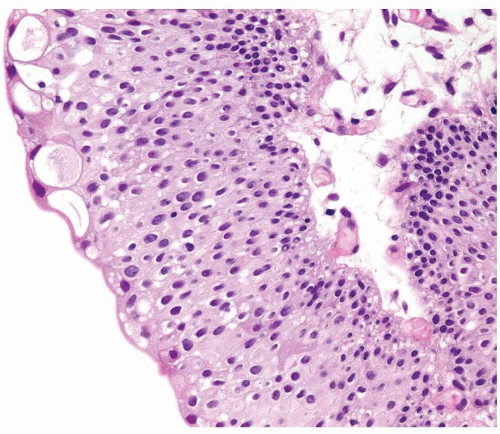
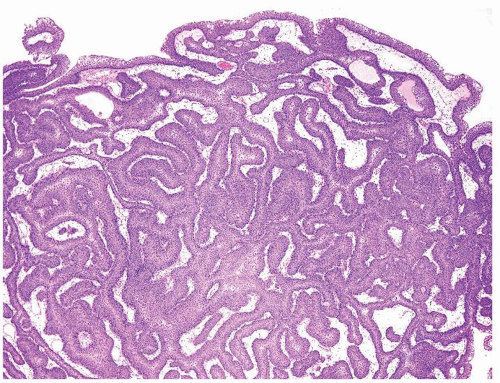
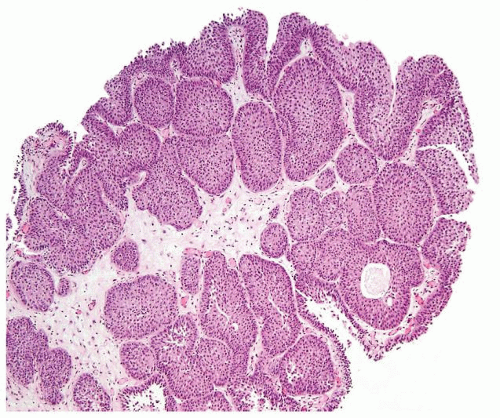
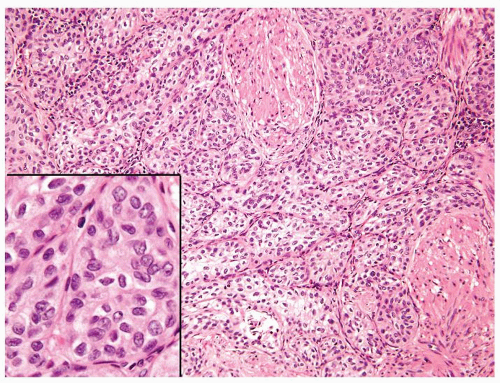
Ranges in size but can be large requiring multiple slides to totally submit
Usually discrete, nonfused papillae but some may be fused
Often with a prominent inverted growth pattern
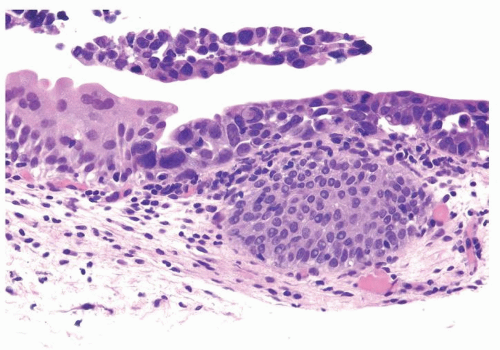
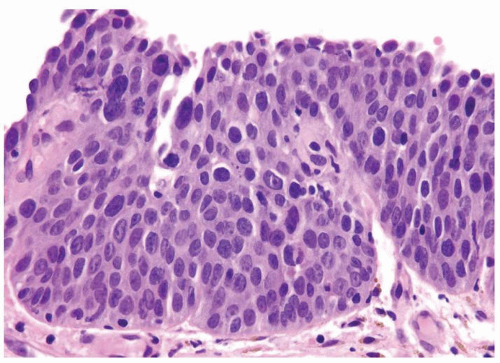
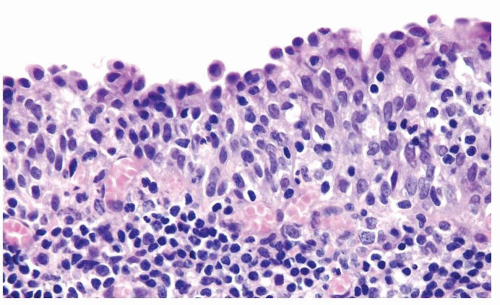
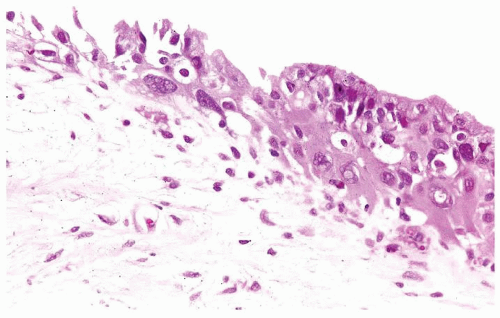
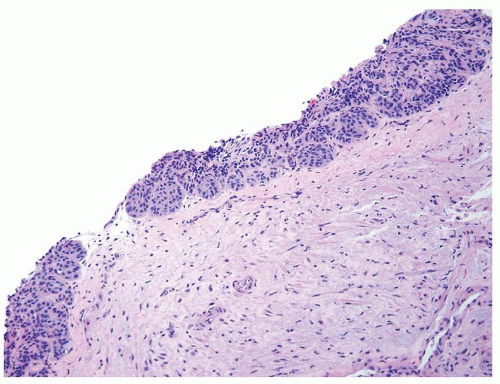
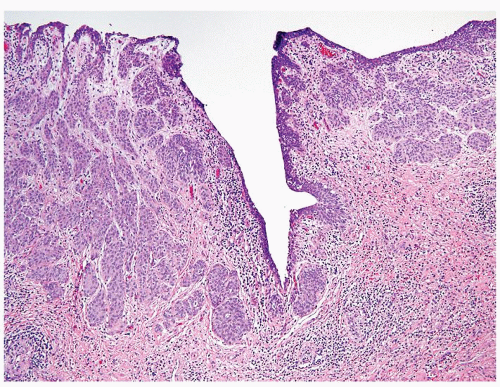
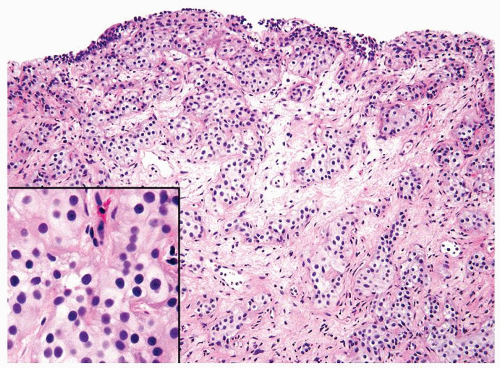
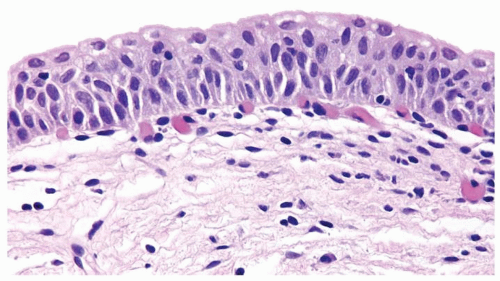
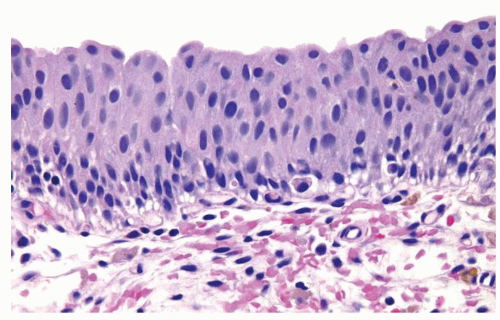
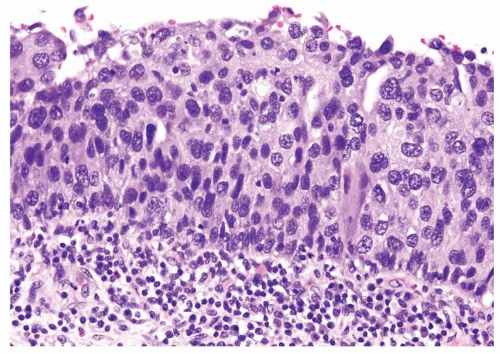
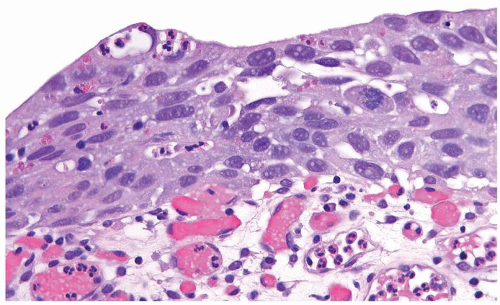
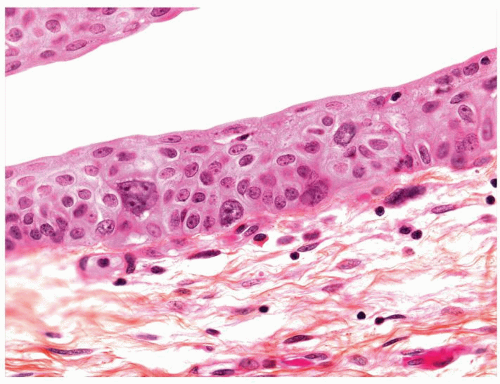
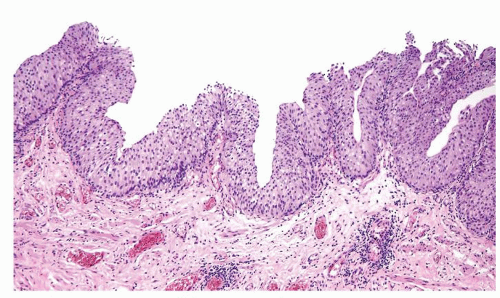
The urothelium is typically overtly thickened
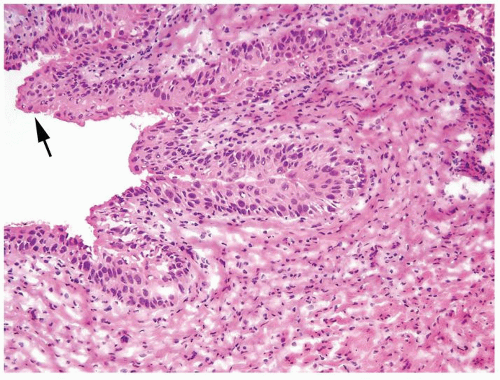
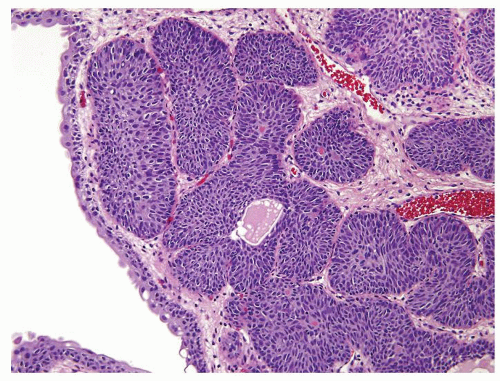
Polarity maintained although may not appear quite as regular as in PUNLMP
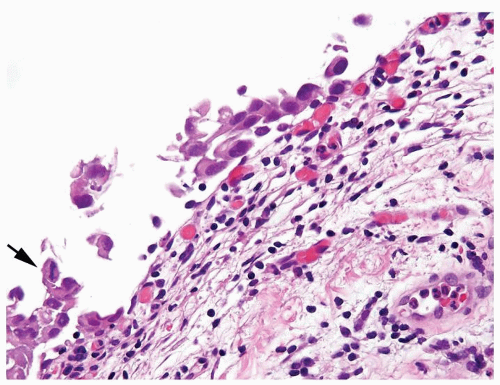
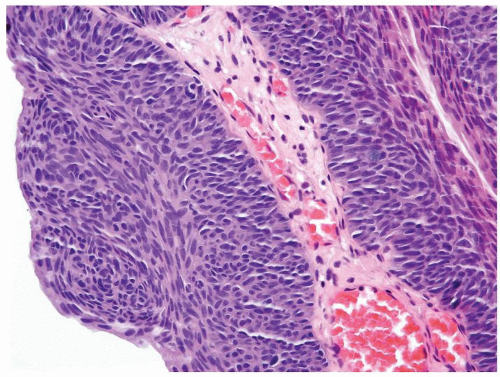
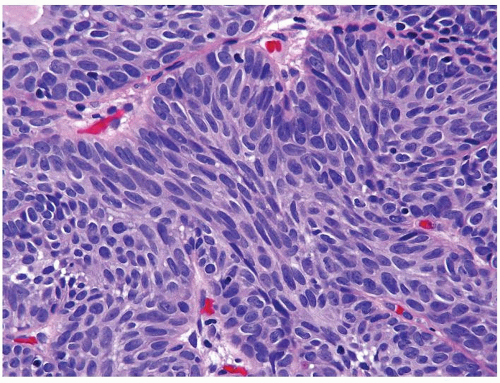
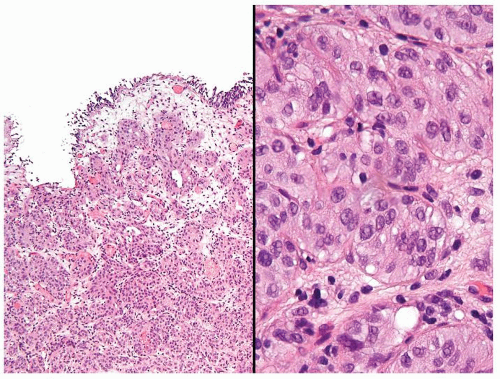
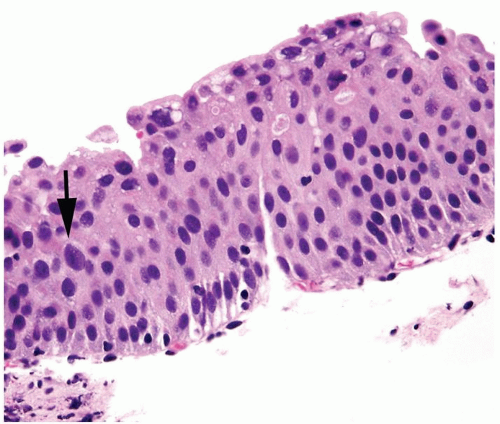
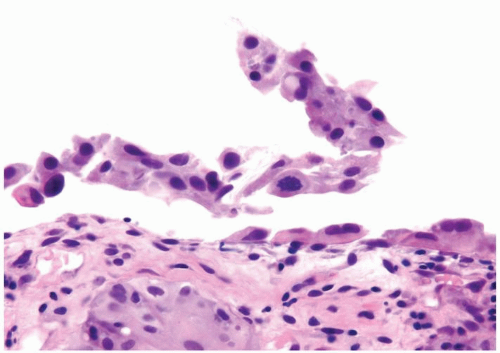
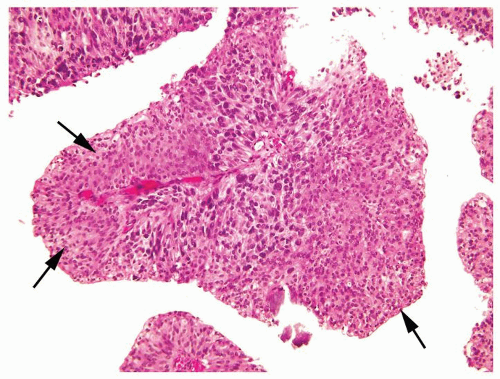
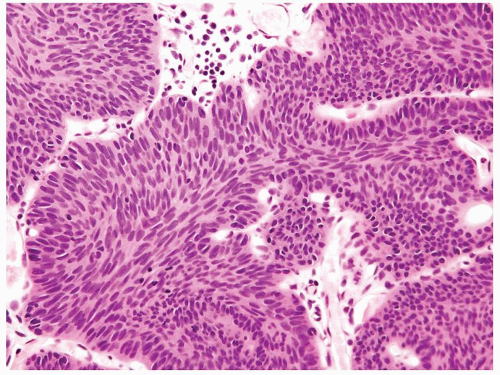
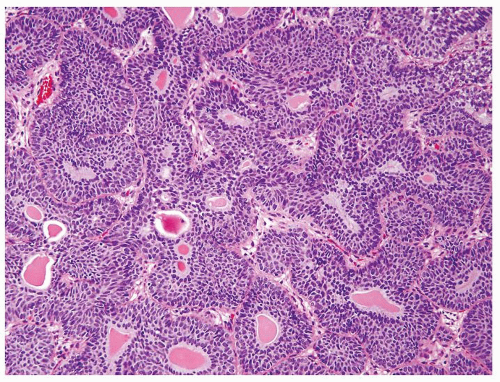
Cytology is mostly normal with scattered slightly enlarged hyperchromatic nuclei that stand out even at lower magnification. Nuclear grooves not as common
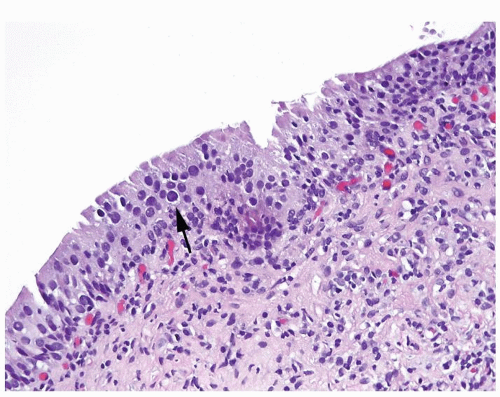
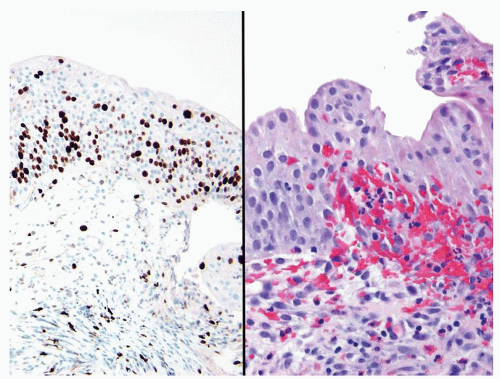
Scattered larger hyperchromatic nuclei with minimal variation in shape. Lacks the uniformity of PUNLMP (Figs.3.10.5, 3.10.6, 3.10.7, 3.10.8)
Nucleoli range from indistinct to small but visible
Mitotic figures range from uncommon to scattered seen at all layers of the urothelium
|
Special studies |
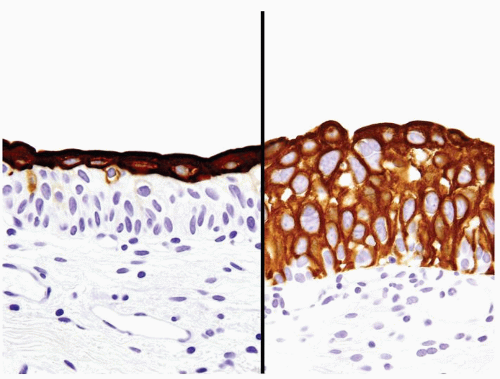
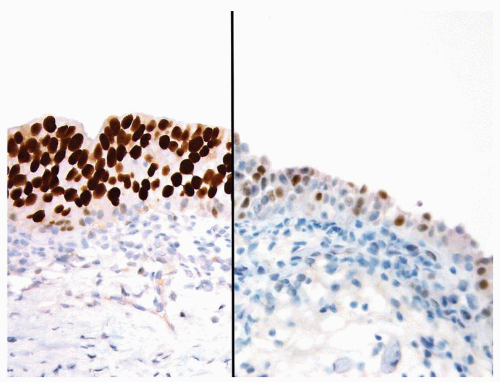
Not helpful in this differential |
Not helpful in this differential |
Treatment |
Typically, resected by TUR and not treated with adjuvant therapy. Followed for life with routine cystoscopy |
Typically, resected by TUR with immediate instillation of intravesical chemotherapy recommended even for lesions with low risk of recurrence. Intravesical BCG not used for initial therapy, yet potentially used in patients with recurrent low-grade papillary urothelial carcinoma. Followed for life with routine cystoscopy |
Prognosis |
At 5 and 15 y, 10% and 30% risk of recurrence, respectively, typically as PUNLMP but occasionally as low-grade carcinoma. Only rare cases recur as higher-grade cancer with invasion. No deaths due to bladder cancer |
At 5 and 15 y, 30% and 40% risk of recurrence, respectively, typically as low-grade carcinoma but occasionally as higher-grade carcinoma. Higher rate of recurrence vs. PUNLMP. 10% of cases recur as higher-grade cancer with invasion, higher rate than in PUNLMP. 1%-2% of patients eventually die due to bladder cancer |