Anthrax
Anthony J. Carbone
NAME OF AGENT
DISEASE: ANTHRAX; ORGANISM: BACILLUS ANTHRACIS.
THEORETICAL AND SCIENTIFIC BACKGROUND
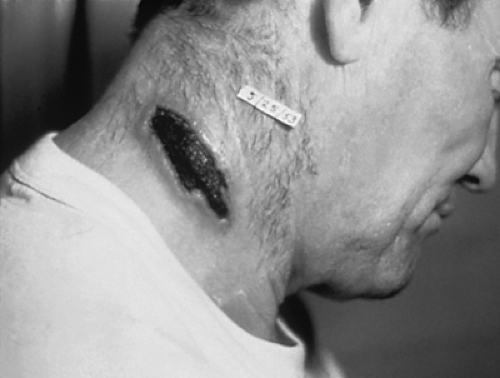
Anthrax is a zoonotic disease caused by the bacterium Bacillus anthracis. Its name is derived from a Greek word for coal, anthrakis, because the disease causes coal-like skin lesions in humans (Fig. 6-1) (1). The disease has been described since antiquity. The biblical fifth plague of ancient Egypt (circa 1500 B.C.) is believed to have been caused by anthrax (2). Virgil recorded a description of anthrax in 25 B.C. (3), and the disease was known as the “Black Bane” during the Middle Ages (4). Robert Koch used anthrax to demonstrate the microbial origin of disease in 1876 (5). Soon after, John Bell recognized Bacillus anthracis as the cause of woolsorter’s disease (inhalational anthrax) and was instrumental in establishing wool disinfection procedures, dramatically reducing the incidence of occupational disease (6). Anthrax is also the first disease after smallpox for which an effective vaccine was developed [by William Greenfield in 1880 (7), followed by Louis Pasteur’s heat-cured vaccine in 1881 (2)].
EPIDEMIOLOGY OF NATURALLY OCCURRING ANTHRAX
Anthrax is a naturally occurring disease that affects herbivores (primarily cattle, sheep, and goats) that become infected after grazing on soil contaminated with B. anthracis spores (9). The animals develop gastrointestinal anthrax followed by systemic dissemination and death (1). Humans generally acquire the disease following contact with anthrax
infected animals or contaminated animal products. Human-to-human transmission has not been described (2,9,10,12).
infected animals or contaminated animal products. Human-to-human transmission has not been described (2,9,10,12).
Anthrax occurs globally and is most common in agricultural regions with inadequate anthrax control programs for livestock. The disease has been reported in over 80 countries. Human cases are rare, but they have been reported in Africa, the Middle East, Asia, Southern and Eastern Europe, the Caribbean, and the Americas (13,14). Cutaneous anthrax is the most common naturally occurring form of anthrax with an estimated 2,000 cases annually worldwide (11). Turkey reports over 400 human cases annually, mostly in the form of cutaneous anthrax (15). The United States experiences rare outbreaks in cattle along the “anthrax belt” of the Great Plains and reported only six human cases between 1988 and 2001 (16,17). Inhalational anthrax is rare with only 18 cases reported in the United States during the 20th century; most of these cases were attributed to occupational exposure among goat hair mill and wool or tannery workers (18,19). The largest reported epidemic of human anthrax occurred in Zimbabwe with over 6,000 cases, virtually all cutaneous, with approximately 100 fatalities reported between 1978 and 1980. The outbreak was attributed to a lapse of established public health and veterinary practices during the Rhodesian Civil War (12,20). Gastrointestinal anthrax is uncommon, but probably underreported. Outbreaks of gastrointestinal anthrax are continually reported in Africa and Asia following ingestion of insufficiently cooked contaminated meat (2).
Deliberate Transmission
Anthrax has long been recognized as a potential biological weapon. The Japanese Imperial Army experimented with anthrax on prisoners of war and Chinese civilians during World War II (21,22). The U.S. military weaponized anthrax spores in the 1950s and 1960s before its offensive biological weapons program was terminated (23). The Soviet Union produced and stockpiled thousands of tons of weaponized anthrax beginning in 1973 and continuing until the fall of the Soviet Union in 1991 (24). One Soviet biological weapons factory, located in Sverdlovsk, USSR (present day Ekaterinsburg, Russia), accidentally released a small amount of militarized anthrax spores in 1979, resulting in 68 reported fatalities. Soviet officials claimed the fatalities were due to gastrointestinal anthrax from tainted meat; however, local pathologists identified the causes of death as inhalational anthrax on autopsy (25,26,27,28,29). In 1995, Iraq admitted to having produced 8,500 liters of anthrax but declared that they destroyed all biological weapons shortly after the start of the Persian Gulf War in 1991 (30,31). The Japanese cult Aum Shinrikyo sprayed anthrax from the rooftop of their Tokyo headquarters in 1993 in an act of terrorism, which failed because the group unwittingly used a benign vaccine strain (32,33). In September 2001, an unknown terrorist mailed threatening letters containing anthrax spores to television news anchor Tom Brokaw, and U.S. Senators Tom Daschle and Patrick Leahy, through the U.S. Postal Service. When it was over, 23 individuals had contracted anthrax—10 with inhalational anthrax and the remainder with cutaneous anthrax. Five of the 23 (22%) victims died as a result of the anthrax infection; all five had been diagnosed with inhalational anthrax (34,35,36,37).
Microbiology
Bacillus anthracis is an aerobic, spore-forming, nonmotile, encapsulated, gram-positive rod (Fig. 6-2). The natural reservoir for B. anthracis is the soil in the spore form of the organism. Anthrax spores germinate into vegetative bacilli when they enter an environment that is rich in amino acids, nucleoside, and glucose, as is found in the blood and tissues of hosts. The vegetative bacilli will form spores when nutrients have been exhausted or when the environment is exposed to ambient air (38,39). Vegetative bacteria cannot survive outside of an animal or human host, whereas the B. anthracis spore is extremely hardy and has been known to survive in soil for decades (40). The vegetative cell is large, measuring 1 to 10 μm by 1 to 1.5 μm. Anthrax spores measure approximately 1 μm (Fig. 6-3). B. anthracis readily grows aerobically on sheep blood agar and is nonhemolytic under these conditions (2). The colonies are large (4 to 5 mm), rough, grayish-white, with a swirling “comet-tail” or “curled-hair” appearance (Fig. 6-4). Several laboratory tests are used to differentiate virulent B. anthracis strains from other innocuous Bacillus species. Unlike other Bacillus species (B. subtillus and B. cereus), B. anthracis is characterized by the absence of hemolysis, motility, growth on phenylethyl alcohol blood agar, gelatin hydrolysis, and salicin fermentation (1,9,2). Microbiologists should be warned of the possibility of B. anthracis in order to run special tests on Bacillus species to rule
out anthrax and to ensure that tests are processed under Biosafety Level 2 (BSL2) conditions.
out anthrax and to ensure that tests are processed under Biosafety Level 2 (BSL2) conditions.
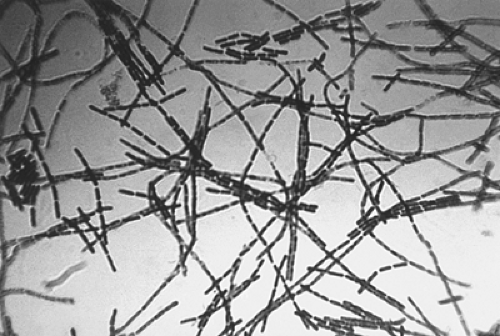 Figure 6-2. Photomicrograph of Bacillus anthracis using Gram stain technique. Source: CDC-PHIL, date unknown. |
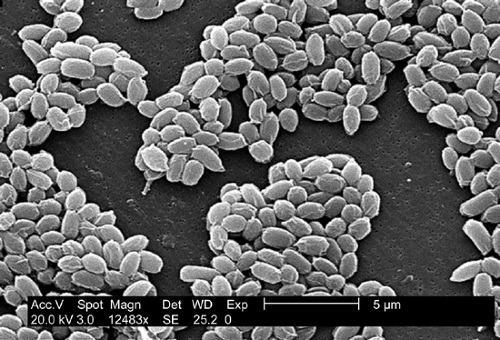 Figure 6-3. An electron micrograph of spores from the Sterne strain of Bacillus anthracis bacteria. Source: CDC-PHIL, 2002. |
Pathogenesis
Anthrax infections are nearly always fatal in animals, but they are not always fatal in humans. Anthrax spores enter the body by inhalation, ingestion, or breaks in the skin where they are phagocytosed by macrophages and carried via lymphatics to regional lymph nodes. The endospores germinate inside the macrophages and become vegetative bacteria. Vegetative bacteria are then released from the macrophages where they multiply in the lymphatic system and enter the bloodstream resulting in massive septicemia (41). The vegetative bacilli express virulence factors that lead to death of the host. The principle virulence factors for B. anthracis are encoded on two plasmids—one involved in the synthesis of an antiphagocytic polypeptide capsule, and the other containing the genes for the synthesis of three proteins it secretes: Protective Antigen (PA), Edema Factor (EF), and Lethal Factor (LF) (1,41,2). Individually these proteins are not cytotoxic; however the combination of PA with LF or EF results in formation of the binary cytotoxins, lethal toxin, and edema toxin, respectively. The PA permits the binding of lethal toxin and edema toxin to the host cell membrane and facilitates their transport across the cell membrane (43). Edema toxin inhibits the neutrophil function and is responsible for the formation of the prominent edema found in anthrax lesions (44,45). Lethal toxin attacks macrophages resulting in the production of reactive oxygen species and the release of the cytokines, tumor necrosis factor-α and interleukin-1α, which lead to shock and sudden death in appropriate concentrations (1,2,47,48,49).
Clinical forms of anthrax
Anthrax manifests itself in three major clinical forms depending upon the route of exposure: cutaneous, gastrointestinal, and inhalational anthrax. Ninety-five percent of human cases of anthrax are reported as cutaneous (1,2,48). The remaining cases tend to be inhalational from occupational exposure to contaminated animals and animal products. Gastrointestinal anthrax is rare, and no cases have been reported in the United States (49). Symptoms and incubation periods vary depending on the route of transmission and the size of the inoculum. In general, symptoms appear within 1 to 7 days of exposure but may develop within hours following a large inoculum; however, it could be as long as 60 days (1,41).
Cutaneous Anthrax
Cutaneous anthrax results from handling contaminated animal tissue or products. Anthrax spores enter the skin through small cuts and abrasions, germinate to the bacillary form, and multiply. Germination takes place in macrophages, and toxins release results in edema and tissue necrosis, with little or no purulence (1). Cutaneous anthrax usually remains local, although if untreated, the disease enters the lymphatics and becomes systemic in 5% to 20% of cases (50). Cutaneous anthrax has a mortality rate of 10% to 20% for untreated cases and is usually less than 1% with treatment (1,2).
Gastrointestinal Anthrax
Gastrointestinal anthrax is the least common, or more likely, the least reported of the three clinical forms of anthrax. Gastrointestinal anthrax is contracted after eating meat contaminated with B. anthracis spores. The spores can infect the system anywhere along the gastrointestinal tract and cause two distinct syndromes based upon the primary site of infection: abdominal and oropharyngeal anthrax. Autopsies suggest that the initial site of infection is most commonly the terminal ileum or cecum (28). The spores germinate into the vegetative form and migrate to the mesenteric and other regional lymph nodes. The bacilli multiply in the lymph nodes resulting in bacteremia and death. The case fatality rate for gastrointestinal anthrax is not precisely known, but it is estimated to be 25% to 60% (9,51). Gastrointestinal anthrax could potentially be the result of an act of bioterrorism. The Japanese Imperial Army’s secret biological warfare program attempted to poison children with chocolate tainted with anthrax spores during World War II (52). The apartheid government of South Africa was found to have developed biological weapons and attempted to poison children with anthrax-containing chocolate (53).
Inhalational Anthrax
Inhalational anthrax develops after the deposition of spore-bearing particles, measuring 1 to 5 μm, into alveolar spaces. The minimum infectious inhaled dose for anthrax is not known for humans, but in nonhuman primate studies it has ranged from 2,500 to 80,000 spores (18,54,55). Inhaled spores are ingested by pulmonary macrophages and are carried to hilar and mediastinal lymph nodes where they germinate and multiply. Although the lung is the initial site of deposition of spores, typical bronchopneumonia does not occur with inhalational anthrax. Instead, multiplying bacilli in the hilar and mediastinal lymph nodes release toxins producing hemorrhagic mediastinitis (27,41). Systemic disease results when bacilli release toxins into the bloodstream causing systemic hemorrhage, edema, and necrosis. The mortality rate for untreated inhalational anthrax reaches 100%. The mortality rate can be reduced if appropriate antibiotic therapy is initiated within 48 hours of exposure, but it may still be as high as 90% (1,2,23,2). The mortality rate for the 2001 anthrax attack was 45% with therapy (10,36).
SIGNS AND SYMPTOMS
Cutaneous Anthrax. Cutaneous anthrax is painless, does not involve a disseminated rash, and results in a localized black eschar that gives the disease its name. Cutaneous anthrax usually occurs following the deposition of spores into skin with previous cuts or abrasions (9,11,56). Areas of exposed skin, such as the hands, arms, face, and neck, are the most frequently affected. Of the victims of the 2001 anthrax attacks who contracted cutaneous anthrax, skin trauma was not noted (57). The incubation period for cutaneous anthrax ranges from 1 to 12 days, but it usually develops within a day (2,11,26). The primary lesion is a painless, pruritic macule or papule resembling an insect bite that develops at the site of infiltration. The lesions enlarge, vesiculate, and often become hemorrhagic. By the second day, the vesicle usually ruptures to form a round, depressed ulcer. Within 1 to 2 days, smaller (1- to 3-mm) satellite vesicular lesions often surround the papule. These lesions are nonpurulent and contain clear or serosanguinous fluid that reveals numerous large bacilli on Gram stain. A painless, depressed, black eschar forms over the lesion and is often associated with significant localized, gelatinous, nonpitting edema. The edema may become massive, especially with lesions involving the face and neck. The eschar dries and falls off within the next 1 to 2 weeks, often without leaving a permanent scar. Lymphangitis and painful lymphadenopathy are often present with associated systemic symptoms. Antibiotic therapy does not appear to change the development of the skin lesion and eventual eschar, but it does decrease the incidence of systemic disease (11). The mortality rate without antibiotics is as high as 20% due to systemic complications. Death with appropriate antibiotic therapy is rare. The differential diagnosis of ulceroglandular lesions includes anti-phospholipid antibody syndrome, brown recluse spider bite, coumadin/heparin necrosis, cutaneous leishmaniasis, cutaneous tuberculosis, ecthyma gangrenosum, glanders, leprosy, mucormycosis, orf, plague, rat bite fever, rickettsial pox, staphylococcal/streptococcal ecthyma, tropical ulcer, tularemia, and typhus. The differential diagnosis for ulceroglandular syndromes includes cat scratch fever, chancroid, glanders, herpes simplex infection, lymphogranuloma venereum, melioidosis, plague, staphylococcal and streptococcal adenitis, tuberculosis, and tularemia (58).
Gastrointestinal Anthrax
Gastrointestinal anthrax develops after eating insufficiently cooked meat contaminated with large numbers of vegetative anthrax bacilli or spores and includes two distinct syndromes: abdominal and oropharyngeal anthrax (11). The symptoms of gastrointestinal anthrax appear 1 to 7 days after ingesting contaminated food and depend upon the site of infection along the alimentary canal.
Abdominal anthrax
The disease usually presents with fever, malaise, nausea, vomiting, and anorexia. The disease rapidly progresses to severe, bloody diarrhea and signs suggestive of acute abdomen and/or sepsis (1,48,56,59,60,61). The primary intestinal lesions are ulcerative and usually occur in the terminal ileum or cecum (62). Gastric ulcers may be associated with hematemesis or coffee-ground emesis (48). Constipation and diarrhea have both been described, and stools are usually melenic or blood-tinged (41). Hemorrhagic mesenteric lymphadenitis and massive ascites are often present (60). Advanced disease may be similar to the sepsis syndrome occurring in late inhalational and cutaneous anthrax. Morbidity is due to blood loss, fluid and electrolyte imbalances, or subsequent shock. Death from intestinal perforation or toxemia occurs 2 to 5 days after the onset of disease (41). Early diagnosis of gastrointestinal anthrax is difficult and mortality may be greater than 50% (62).
Oropharyngeal anthrax
Deposition and germination of spores in the oropharynx result in a subset of gastrointestinal anthrax known as oropharyngeal anthrax. Patients present complaining of a severe sore throat, fever, dysphagia, and occasionally respiratory distress (21,48). Other clinical features include toxemia, inflammatory lesions of the oral cavity or oropharynx, and marked cervical lymphadenopathy with edema of the soft tissues of the cervical area (62). Swelling may be severe enough to compromise the airway. In an outbreak of anthrax in April 1982 in northern Thailand, 24 cases of oropharyngeal anthrax were reported (63). All of the afflicted had recently eaten water buffalo meat. All but one required hospitalization, and three of the 24 (12.4%) patients died despite hospitalization and antibiotic therapy (64). All of the patients sought medical attention because of painful neck swelling, and all but one complained of fever. Other common symptoms included sore throat, dysphagia, and hoarseness. The neck swelling was due to marked cervical lymphadenopathy and soft tissue edema. Early lesions are edematous. Within 7 days, central necrosis and ulceration develop and form whitish patches. By the second week, the patch develop into a pseudomembrane covering the ulcer similar to diphtheria (62).
Inhalational Anthrax
The classic clinical course of inhalational anthrax is one of a biphasic illness. The incubation period for inhalational anthrax typically ranges from 1 to 7 days, but may develop as quickly as hours or as long as 60 days following exposure depending upon the size of the inoculum (1,2,11,27,41). The initial phase begins with a nonspecific illness characterized by fever, chills, headache, malaise, myalgias, weakness, nonproductive cough, and some chest and/or abdominal discomfort and may last from hours to a few days (2,11,18,57,65). The initial phase may be followed by a short latent period lasting hours to a few days. The second phase manifests abruptly with sudden high fever, diaphoresis, acute dyspnea, cyanosis, chest discomfort, and shock (1,2,26,27,28,41). Stridor may be present due to extrinsic obstruction of the trachea by lymphadenopathy, mediastinal widening, and subcutaneous edema of the chest and neck (11). The disease progresses rapidly with hypotension, hypothermia, shock, and death occurring within 24 to 36 hours. The differential diagnosis for inhalational anthrax includes influenza and influenza-like illnesses from other causes. The CDC has published the results of a study comparing signs and symptoms of patients diagnosed with inhalational anthrax, laboratory-confirmed influenza, and influenza-like illnesses from other causes (66). In this study, sore throat and rhinorrhea were common in influenza and influenza-like illnesses, whereas they were rare in inhalational anthrax (sore throat—20%, rhinorrhea—10%). Shortness of breath was prevalent in the patients with inhalational anthrax (80%) compared to only 8% in influenza, and 6% for influenza-like illnesses from other causes. Chest discomfort or
pleuritic chest pain was present in 80% of the anthrax patients and 35% of the influenza patients but only 23% of those suffering from influenza-like illnesses other than influenza (66).
pleuritic chest pain was present in 80% of the anthrax patients and 35% of the influenza patients but only 23% of those suffering from influenza-like illnesses other than influenza (66).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree