Objectives
- Identify the 3 families of anterior pituitary hormones and their main structural differences.
- Understand the mechanisms that regulate anterior pituitary hormone production and describe the actions of tropic hormones on target organs.
- Diagram the short-loop and long-loop negative feedback control of anterior pituitary hormone secretion.
- Predict the changes in secretory rates of hypothalamic anterior pituitary and target gland hormones caused by oversecretion or undersecretion of any of these hormones or receptor deficit for any of these hormones.
- Explain the importance of pulsatile and diurnal hormone secretion.
Anterior Pituitary Gland: Introduction
![]() The anterior pituitary, or adenohypophysis, plays a central role in the regulation of endocrine function through the production and release of tropic hormones (Figure 3–1). The function of the anterior pituitary, and thereby the production of tropic hormones, is under hypothalamic regulation by the hypophysiotropic neuropeptides released in the median eminence, as discussed in Chapter 2 and summarized in Table 3–1. The tropic hormones produced by the anterior pituitary are released into the systemic circulation, from where they reach their target organs to produce a physiologic response, most frequently involving the release of a target organ hormone (see Figure 3–1). The hormones produced by the target organs affect anterior pituitary function as well as the release of hypophysiotropic neuropeptides, maintaining an integrated feedback control system of endocrine function (see Chapter 1, Figure 1–12).
The anterior pituitary, or adenohypophysis, plays a central role in the regulation of endocrine function through the production and release of tropic hormones (Figure 3–1). The function of the anterior pituitary, and thereby the production of tropic hormones, is under hypothalamic regulation by the hypophysiotropic neuropeptides released in the median eminence, as discussed in Chapter 2 and summarized in Table 3–1. The tropic hormones produced by the anterior pituitary are released into the systemic circulation, from where they reach their target organs to produce a physiologic response, most frequently involving the release of a target organ hormone (see Figure 3–1). The hormones produced by the target organs affect anterior pituitary function as well as the release of hypophysiotropic neuropeptides, maintaining an integrated feedback control system of endocrine function (see Chapter 1, Figure 1–12).
Figure 3–1.
Anterior pituitary hormones, target organs, and physiologic effects. Thyroid-stimulating hormone (TSH) stimulates the thyroid gland to produce and release thyroid hormones that regulate growth, differentiation, and energy balance. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) stimulate gonadal production of sex steroids, which mediate reproductive function and behavior. Adrenocorticotropic hormone (ACTH) stimulates the adrenal glands to produce steroid hormones, which regulate water and sodium balance, inflammation, and metabolism. Prolactin (Prl) stimulates breast development and milk production. Growth hormone (GH) exerts direct effects on tissue growth and differentiation and indirect effects through the stimulation of insulin-like growth factor 1 production, which mediates some of the growth and differentiation effects of GH.
| Anterior pituitary cells | Hypothalamic factor | Pituitary hormone produced |
|---|---|---|
| Lactotrophs | Dopamine | Prolactin |
| Corticotrophs | CRH | POMC: ACTH, β-LPH, α-MSH, β-endorphin |
| Thyrotrophs | TRH | Thyroid-stimulating hormone |
| Gonadotrophs | GnRH | LH and FSH |
| Somatotrophs | GHRH | Growth hormone |
Functional Anatomy
The pituitary, or hypophysis, consists of an anterior and a posterior lobe that differ from one another in their embryologic origin, mode of development, and structure. The anterior lobe, also known as the adenohypophysis, is the larger and consists of a pars anterior and a pars intermedia, or intermediate lobe, separated from each other by a narrow cleft, the remnant of Rathke’s pouch. The pars intermedia is of minor importance in human physiology. The anterior pituitary is a highly vascularized structure consisting of epithelial cells derived from the ectodermal lining of the roof of the mouth. The pituitary cells that line the capillaries produce the tropic hormones: adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH), growth hormone (GH), prolactin, and the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (see Figure 3–1). All of these hormones are released into the systemic circulation.
The cells of the anterior pituitary are named according to the hormone that they produce. According to their specific distribution, they may be more or less susceptible to traumatic injury. For example, the gonadotrophs and somatotrophs (GH-producing cells) are more numerous in the posterolateral region of the anterior pituitary, making them vulnerable to mechanical damage of the pituitary. The corticotrophs (ACTH-producing cells) and the thyrotrophs (TSH-producing cells) are located predominantly in the anteromedial region, making them more resilient to traumatic injury. The lactotrophs (prolactin-producing cells) are dispersed throughout the pituitary, and this too is a resilient cell population. The posterior pituitary is of nervous origin. It consists of unmyelinated nerve fibers and axon terminals of magnocellular hypothalamic neurons, with bodies located primarily in the supraoptic and paraventricular hypothalamic nuclei. The neurohormones released from the posterior pituitary have been discussed in Chapter 2. This chapter will focus on the endocrine function of the anterior pituitary.
Hypothalamic Control of Anterior Pituitary Hormone Release
![]() The production of pituitary tropic hormones is under direct regulation by the hypothalamic neurohormones released from neuronal terminals in the median eminence. The neurohormones are delivered to the anterior pituitary through a specialized capillary network, described in Chapter 2 and illustrated in Figure 2–2. The hypothalamic neuropeptides travel down the long hypophysial portal veins to the anterior pituitary, where they bind to specific cell surface G protein–coupled receptors and activate intracellular second-messenger cascades, resulting in the release of pituitary hormone from the respective target cells.
The production of pituitary tropic hormones is under direct regulation by the hypothalamic neurohormones released from neuronal terminals in the median eminence. The neurohormones are delivered to the anterior pituitary through a specialized capillary network, described in Chapter 2 and illustrated in Figure 2–2. The hypothalamic neuropeptides travel down the long hypophysial portal veins to the anterior pituitary, where they bind to specific cell surface G protein–coupled receptors and activate intracellular second-messenger cascades, resulting in the release of pituitary hormone from the respective target cells.
The responsiveness of the anterior pituitary to the inhibitory or stimulatory effects of hypophysiotropic neurohormones can be modified by several factors, including hormone levels, negative feedback inhibition, and circadian rhythms as discussed in Chapter 1. The release of anterior pituitary hormones is cyclic in nature, and this cyclic pattern of hormone release is governed by the nervous system. Most rhythms are driven by an internal biologic clock located in the hypothalamic suprachiasmatic nucleus; this clock is synchronized or entrained by external signals such as light and dark periods. Both sleep and circadian effects interact to produce the overall rhythmic pattern of pituitary hormone release and the associated responses. Some of the 24-hour hormonal rhythms depend on the circadian clock (ie, ACTH, cortisol, and melatonin) and some are sleep related (ie, prolactin, GH, and TSH). For example, GH secretion is influenced by the first slow-wave sleep episode at the beginning of the night. Pulses of prolactin and GH are positively linked to increases in delta-wave activity, present during the deepest phases of sleep and occurring primarily during the first third of the night. Pulses of TSH and cortisol are related to superficial phases of sleep.
![]() Although the regulation of the patterns of hormone release is not well understood, it is clear that the respective patterns of anterior pituitary hormone release play a crucial role in achieving their physiologic effects and, thus, in maintaining homeostasis. The importance of this regulation has become evident because constant or continuous exogenous hormone administration produces effects that differ from the hormone’s natural physiologic effects. These observations have highlighted the importance of trying to simulate, as much as possible, the endogenous cyclic patterns of hormone release when giving hormone replacement therapy to a patient. In addition, disruption of the cyclic patterns of hormone release has been identified in disease states and is thought to play an important role in the impaired endocrine function that occurs with aging. Disruption of circadian rhythms leads to symptoms of fatigue, disorientation, altered hormone profiles, and higher morbidity. Therefore, the natural cyclic pattern of hypothalamic, pituitary, and target organ hormone release is of central importance to normal endocrine function.
Although the regulation of the patterns of hormone release is not well understood, it is clear that the respective patterns of anterior pituitary hormone release play a crucial role in achieving their physiologic effects and, thus, in maintaining homeostasis. The importance of this regulation has become evident because constant or continuous exogenous hormone administration produces effects that differ from the hormone’s natural physiologic effects. These observations have highlighted the importance of trying to simulate, as much as possible, the endogenous cyclic patterns of hormone release when giving hormone replacement therapy to a patient. In addition, disruption of the cyclic patterns of hormone release has been identified in disease states and is thought to play an important role in the impaired endocrine function that occurs with aging. Disruption of circadian rhythms leads to symptoms of fatigue, disorientation, altered hormone profiles, and higher morbidity. Therefore, the natural cyclic pattern of hypothalamic, pituitary, and target organ hormone release is of central importance to normal endocrine function.
Hormones of the Anterior Pituitary
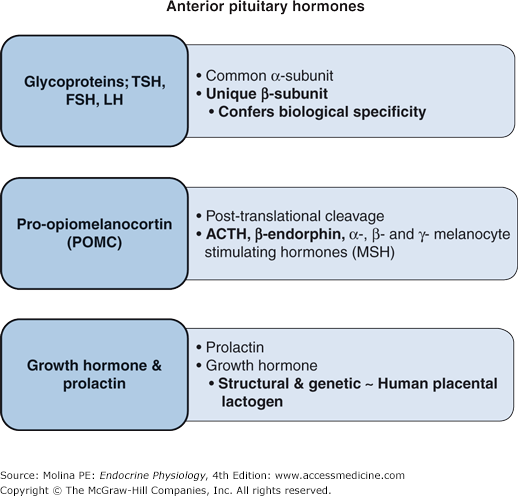
The hormones of the anterior pituitary can be classified into 3 families: the glycoproteins, those derived from proopiomelanocortin (POMC), and those belonging to the GH and prolactin family (Figure 3–2).
Figure 3–2.
Classification of anterior pituitary hormones. Thyroid-stimulating hormone (TSH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) are glycoproteins with very similar structures. Proopiomelanocortin (POMC) is a polypeptide hormone that is posttranslationally cleaved to adrenocorticotropin (ACTH), β-endorphin, and melanocyte-stimulating hormones. Growth hormone and prolactin are structurally similar to human placental lactogen.
Glycoprotein hormones are among the largest hormones known to date. They include TSH, FSH, LH, and human chorionic gonadotropin (HCG) produced by the placenta. These hormones are heterodimeric glycoproteins consisting of a common α-subunit and a unique β-subunit, which confers the biologic specificity of each hormone.
TSH is a glycoprotein synthesized and secreted from thyrotrophs of the anterior pituitary gland. Thyrotrophs synthesize and release TSH in response to thyrotropin-releasing hormone (TRH) stimulation. TRH is synthesized in the paraventricular nuclei of the hypothalamus, predominantly by parvicellular neurons, and is released from nerve terminals in the median eminence. TRH binds to a Gq/11 protein–coupled receptor, which activates phospholipase C, leading to increased phosphoinositide turnover, calcium mobilization, and release of TSH into the circulation. TSH binds to a Gs protein–coupled receptor in the thyroid gland, activating adenylate cyclase and leading to increased intracellular cyclic 3′,5′-adenosine monophosphate (cAMP) formation and stimulation of the protein kinase A signaling pathway. TSH stimulates all the events involved in thyroid hormone synthesis and release (see Chapter 4). In addition, it acts as a growth and survival factor for the thyroid gland. The release of TSH from the anterior pituitary gland is under negative feedback inhibition by thyroid hormone, particularly triiodothyronine, as discussed in detail in Chapter 4.
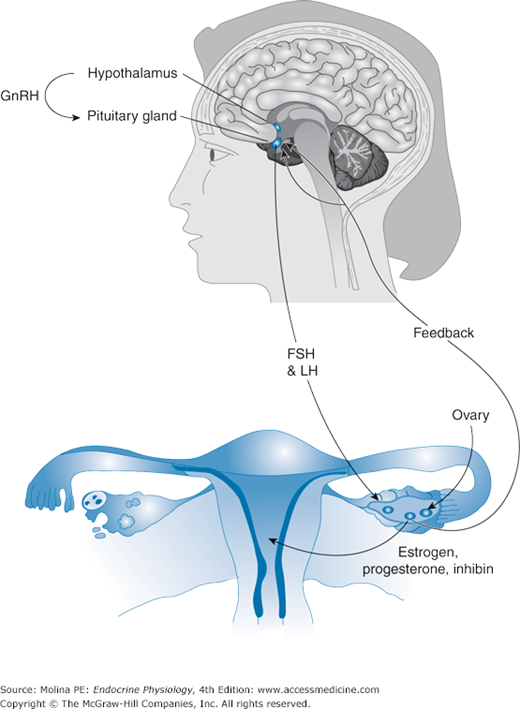
The gonadotropic hormones LH and FSH are synthesized and secreted by gonadotrophs of the anterior pituitary in response to stimulation by gonadotropin-releasing hormone (GnRH). Most of the gonadotrophs produce both LH and FSH; with a fraction of the gonadotroph population producing LH or FSH exclusively. GnRH is synthesized and secreted by the hypothalamus in a pulsatile manner. GnRH binds to the GnRH Gq/11 protein–coupled receptor on pituitary gonadotrophs and produces activation of phospholipase C, leading to phosphoinositide turnover and Ca2+ mobilization and influx. This signaling cascade increases the transcription of the FSH and LH α-subunit and β-subunit genes and increases the release of FSH and LH into the circulation.
FSH and LH exert their physiologic effects on the testes and ovaries by binding to Gs protein–coupled receptors and activating adenylate cyclase. Among the target cells for gonadotropins are ovarian granulosa cells, theca interna cells, testicular Sertoli cells, and Leydig cells. The physiologic responses produced by the gonadotropins include stimulation of sex hormone synthesis (steroidogenesis), spermatogenesis, folliculogenesis, and ovulation. Therefore, their central role is the control of reproductive function in both males and females. GnRH controls the synthesis and secretion of both FSH and LH by the pituitary gonadotroph cell. Gonadotropin synthesis and release, as well as differential expression, is under both positive and negative feedback control by gonadal steroids and gonadal peptides (Figure 3–3). Gonadal hormones can decrease gonadotropin release both by decreasing GnRH release from the hypothalamus and by affecting the ability of GnRH to stimulate gonadotropin secretion from the pituitary itself. Estradiol enhances LH and inhibits FSH release, whereas inhibins A and B, gonadal glycoprotein hormones, reduce FSH secretion (see Chapter 9).
Figure 3–3.
Feedback regulation of pituitary hormone release. Hypothalamic neurohormones (eg, gonadotropin-releasing hormone) stimulate the anterior pituitary to produce and release tropic hormones (eg, follicle-stimulating hormone and luteinizing hormone). Tropic hormones bind to receptors in target organs and elicit a physiologic response. In most cases, the response involves the production of a target organ hormone, which, in turn, mediates physiologic effects at the target organ (eg, uterus). In addition, the target organ hormone is involved in feedback mechanisms (negative or positive) that regulate the production and release of the tropic hormone and the hypothalamic factor that regulates pituitary hormone release.
The complexity of the regulation of synthesis and release of anterior pituitary hormones is best illustrated by the cyclic nature of FSH and LH release. The pattern of GnRH pulses changes during the menstrual cycle in women, as summarized in Table 3–2 and discussed in detail in Chapter 9. During the luteal to follicular phase transition, pulses of GnRH release occur every 90–120 minutes, and FSH secretion predominates. In the mid-to-late follicular phase, GnRH frequency increases to 1 pulse every 60 minutes, favoring LH secretion over FSH. After ovulation, ovarian progesterone production predominates. Progesterone increases hypothalamic opioid activity and slows GnRH pulse secretion. This slower GnRH pulse pattern (1 pulse per 3–5 hours) favors FSH production. However, at the same time, estradiol and inhibin A produced by the corpus luteum inhibit FSH release, leading to increased FSH stores. With involution of the corpus luteum and the sharp decline in estradiol, inhibin A, and progesterone, the frequency of GnRH pulse secretion is increased. In the absence of estradiol and inhibin A (inhibitors of FSH release), a selective FSH release predominates and initiates the next wave of follicular development.
| Phase of menstrual cycle | Gonadal hormones | GnRH pulses | Gonadotropin release |
|---|---|---|---|
| Luteal to follicular transition | Low estradiol, low inhibin | 90–120 min | FSH > LH |
| Mid to late follicular phase | Increasing estradiol and inhibin B | Increased pulsatility; 60 min | LH > FSH |
| Post ovulation | Increased estradiol, inhibin A, and progesterone | Decreased GnRH pulsatility | Increased FSH synthesis; inhibited release |
| Corpus luteum involution | Decreased estradiol, inhibin A, and progesterone | Increased GnRH pulsatility | FSH |
POMC is a precursor pro-hormone produced by the corticotrophs of the anterior pituitary. The production and secretion of POMC-derived hormones from the anterior pituitary are regulated predominantly by corticotropin-releasing hormone (CRH) produced in the hypothalamus and released in the median eminence. CRH binds to a Gs protein–coupled receptor whose actions are mediated through activation of adenylate cyclase and elevation of cAMP production (see Figure 3–3). Two types of remarkably homologous (approximately 70% amino acid identity) CRH receptors have been identified. Both CRH-1 and CRH-2 receptors belong to the family of transmembrane receptors that signal by coupling to G proteins and use cAMP as a second messenger. Stimulation of POMC synthesis and peptide release is mediated by the CRH-1 receptor, which is expressed in many areas of the brain as well as in the pituitary, gonads, and skin. CRH-2 receptors are expressed on brain neurons located in neocortical, limbic, and brainstem regions of the central nervous system and on pituitary corticotrophs and in peripheral tissues (eg, cardiac myocytes, gastrointestinal tract, lung, ovary, and skeletal muscle). The role of CRH-2 receptors is not completely understood.
POMC is posttranslationally cleaved to ACTH; β-endorphin, an endogenous opioid peptide; and α-, β-, and γ-melanocyte-stimulating hormones (MSHs) (see Figure 3–5). The biologic effects of POMC-derived peptides are largely mediated through melanocortin receptors (MCRs), of which 5 have been described. MC1R, MC2R, and MC5R have defined roles in the skin, adrenal steroid hormone production, and thermoregulation, respectively. MC4R is expressed in the brain and has been implicated in feeding behavior and appetite regulation. The role of MC3R is not well defined.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree