Animal Testing and Animal Welfare
The public will choose to believe a simple lie rather than a complex truth.
–Alexis De Tocqueville
Everyone “knows” what animals are, but the word animal may be defined in several ways. The United States Public Health Service defines an animal as “any live vertebrate animal.” This definition draws a line between vertebrates and invertebrates, which is a useful distinction in many situations and serves as a basis to identify the scope of the animal testing issue. Another term used to draw a line between types of animals is sentient (i.e., an animal that can feel; having feelings). One problem with the use of this term is that it is unclear where to draw a distinction between animals that are and are not sentient. The presence of primitive nervous systems that enable simple reflexes to occur do not indicate that the animal can feel.
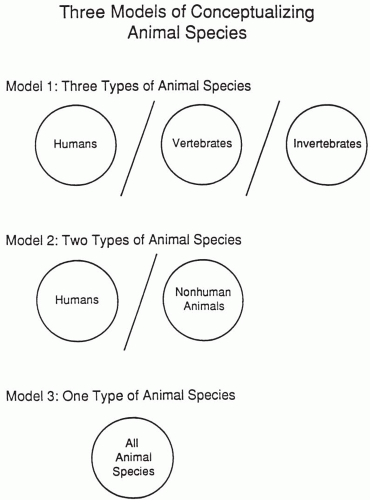
Figure 104.1 presents visual schematics of three ways of conceptualizing all animal species. The models differ based on whether qualitative distinctions are made between humans and vertebrates and between vertebrates and invertebrates. Virtually all representatives of the pharmaceutical industry and the groups that conduct animal experiments conceptually believe in Model 1 or 2, whereas some (or many) animal rights advocates believe in Model 3. Model 3 states that all animal species are qualitatively the same, although whether all invertebrates or nonsentient animals are included in the definition varies among those who would state that Model 3 is correct. It is one thing to say animals are sentient (i.e., responsive to or aware of sensory stimuli) and another to say they have rights. Animals can be said to have interests and therefore humans have some level of moral concern about how they are treated. The moral rights of the human species are based on views expressed by various philosophers, particularly Locke and Kant. Moral consideration of animals is based on their interests. Nonsentient living organisms (e.g., plants) have needs, but not interests in the sense of sentient animals.
The most important point from a pharmaceutical company’s perspective is that there is a distinction between humans and non-humans as illustrated in Models 1 and 2. This chapter is therefore based on the philosophical belief underlying Models 1 or 2.
THE CENTRAL ISSUE ABOUT PROTECTING ANIMAL RIGHTS DURING EXPERIMENTATION
The balance is widely debated about how our society should best protect the environment and its human and animal inhabitants, while at the same time utilizing environmental resources to
improve the status of people. There is general agreement that animal research has played a pivotal role in discovering new drugs and surgical methods that have saved many human lives, lessened human suffering, and advanced scientific knowledge. Part of the price for these advances is that animals have been sacrificed. Most of these animals, however, have been specifically bred for the purpose of experimentation.
improve the status of people. There is general agreement that animal research has played a pivotal role in discovering new drugs and surgical methods that have saved many human lives, lessened human suffering, and advanced scientific knowledge. Part of the price for these advances is that animals have been sacrificed. Most of these animals, however, have been specifically bred for the purpose of experimentation.
Over the past 25 years, the debate over animal welfare has intensified. The extreme position of animal abuse exemplified by dog fighting and cock fighting is abhorred by almost all members of our society. The major issue in conducting research in animals that has arisen over the past 25 years involves the spectrum of scientists ranging from those who want complete freedom to conduct research on animals without any restraints to those who state that all research involving animals should be stopped. While there are some people who adhere to one of these extremes, most scientists and most of society represent the middle ground. Intense debate and even violence have erupted over where a modern society should stand along this spectrum. An important question to ask is this: “What is the correct balance between allowing appropriate research and protecting the welfare of the animals?” This issue is sometimes described as identifying the conditions that must be present for animal experiments to be conducted and how society can ensure appropriate care of the animals when experiments are conducted.
This chapter does not attempt to explore the background, or details of the social, philosophical, or political issues that have focused on this debate. This chapter has a far simpler goal of attempting to present some of the major issues behind the animal testing controversy from a pharmaceutical company’s perspective on procedures that should be followed in assuring that protocols for animal studies are appropriately reviewed, that animals are treated appropriately, and reviewing alternative uses to animals in research.
Primary Uses of Animals in Research
Animals are required in pharmaceutical research to determine if compounds have specific biological activity at pharmacological doses or toxic effects at toxicological doses. Animals are also used to develop new procedures or tests that may assist in improving surgery in humans or testing certain medical devices that cannot be initially evaluated in humans. Animals are also used for behavioral studies and for a wide range of educational purposes (e.g., teaching medical students).
RESPONSIBILITIES OF INSTITUTIONS CONDUCTING ANIMAL STUDIES
Oversight Committee
Current standards as well as laws in many countries require that an oversight committee be established to review and approve all nonhuman animal protocols before they are initiated. These protocols include all studies conducted in toxicology, pharmacology, and other scientific disciplines (e.g., biochemistry, metabolism, pharmacokinetics, microbiology, virology) where animals are used. This or another committee must also be charged with the responsibility of ensuring appropriate care and housing of all animals in the institution.
Guidelines
In the United States, relevant guidelines are described in the 83-page Guide for the Care and Use of Laboratory Animals (US Department of Health and Human Services, National Institutes of Health 1985). Federal laws are identified in an appendix to this booklet, which stresses that professional judgment is essential when applying these guidelines. The laws on which these guidelines are based are the 1985 amendments to the Animal Welfare Act (1966) administered by the US Department of Agriculture and the Health Research Extension Act (1985). The latter act is important for groups funded by the US Public Health Service. These laws mandate a local review (oversight) committee at each institution that uses animals (i.e., the Institutional Animal Care and Use Committee). Such a committee must include a veterinarian experienced in animal care and a person who is unaffiliated with the institution. The functions of this committee are to inspect animal research areas at least twice a year to ensure that relevant guidelines and regulations are being followed, and to review protocols for proposed experiments to ensure humane treatment of animals.
Several European countries also have approved regulations that protect animal welfare and in some cases provide for mandatory licensing of scientists and technicians who operate on or use animals in experiments. A summary of the early experiences with ethical committees for protecting animals in the United Kingdom and Sweden is given by Britt (1983).
Animal Care and Use Committee
Every research institution in the United States that uses animals must have an animal care and use committee. One exception is for institutions that do not receive Public Health Service funding and only use species not covered by the Animal Welfare Act (i.e., mice, rats, and birds). In some countries, an Animal Care and Use Committee is also mandated by law, but if not, institutions should establish such committees. This committee may have a different name, but its function is to oversee the care, humane use, and protection of animal welfare at the facility/institution. Existing regulations in some countries influence the makeup of the committee. It must (should) include a scientist who is experienced in animal research, a veterinarian, a “well-respected” member of the community, and others as required (or desired) by custom or law. Depending on the laws of the country involved and interests of the company, the animal care and use committee could be the same as the oversight committee previously described.
Functions of the Institutional Animal Care and Use Committee include the following:
Establishment (or endorsement) of procedures for the facility/ institution regarding animal care and use
Monitoring of the animal care and use at the institution to ensure that all relevant guidelines and policies are being met
Periodic meetings (e.g., one to four times each year) to discuss issues that arise relating to animal care and use, including (a) certification and training of relevant students, staff, and technical personnel; (b) occupational hazards (e.g., animal bites, disease transmission); (c) protective clothing; (d) protection of staff from hazardous biological, chemical, and physical agents; and (e) supervision of hazardous agents and animal projects
Periodic meetings to review protocols for conducting scientific experiments submitted by institutional staff
Preparation of an annual report on the status of the above points for relevant administrators (in the United States, this would be the US Department of Agriculture and the Public Health Service, which also inspects animal care facilities)
Semiannual program review and facilities inspection
Each pharmaceutical company conducting research in the United States must have an animal use committee that reviews and approves protocols for scientific experiments involving use of animals (in vitro or in vivo). Pharmaceutical companies conducting research using animals in other countries should have such committees. The membership of this group can vary in number, but three to six people are generally sufficient to meet their mandate. Their mandate is to ensure that the methods of animal care, treatment, and handling adhere to principles of the company or institution plus all regulations. This group can also have an oversight responsibility to ensure that animal husbandry and all facilities meet appropriate standards. Individuals who supervise or audit Good Laboratory Practices regulations could also report to the committee on a periodic basis to review their activities and findings. These last few functions go beyond conducting protocol review and an organization may choose to have these activities reviewed by other groups. The point is that attention should be given to ensuring that a specific group is identified that has responsibility for all functions relating to animal well being.
Training
In the United Kingdom and some other countries as well, all scientific staff that use animals must be licensed. In all countries, staff lacking adequate prior experience must be trained by the company. One option is to establish a brief training course to review basic animal behavior and techniques for handling and using animals in research. If anesthetics are to be used, then
appropriate training on that subject should be included as part of the course.
appropriate training on that subject should be included as part of the course.
Table 104.1 Suggested contents of a protocol for experimenting on animals | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree