Advanced Aneurysm Management Techniques: Management of Internal Iliac Aneurysm Disease
W. Anthony Lee
DEFINITION
Iliac aneurysm is defined as an iliac artery whose diameter is 20 mm or more. Iliac aneurysms are present in up to 20% of abdominal aortic aneurysms,1 and common iliac aneurysms occur far more frequently than internal iliac aneurysms.
Isolated iliac aneurysms represent less than 5% of all aortoiliac aneurysms.
External iliac aneurysms are extremely rare and mostly either associated with underlying connective tissue disorders or represent traumatic pseudoaneurysms.
DIFFERENTIAL DIAGNOSIS
Differential diagnoses of iliac aneurysm are limited to true degenerative aneurysms, which are most common; mycotic, traumatic, or surgical pseudoaneurysms; or aneurysmal enlargement of the false lumen from a primary dissection.
PATIENT HISTORY AND PHYSICAL FINDINGS
Most iliac aneurysms are clinically silent (asymptomatic). Rarely, in very thin individuals with large aneurysms, a pulsatile aneurysm may be palpable on physical examination. Even more rarely, a patient being evaluated for hydroureter may be determined to have an iliac aneurysm. Ureteral obstruction in this circumstance derives from perianeurysmal inflammation (similar to retroperitoneal fibrosis) rather than mechanical compression by the aneurysm.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Although a plain abdominal x-ray can detect an aortoiliac aneurysm if there is heavy mural calcification, the most common imaging modalities include ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI).
Thin-cut (1 mm), intravenous contrast-enhanced, spiral CT (CT arteriogram) represents the “gold standard” for diagnosis and anatomic evaluation of abdominal aneurysms. Even in patients with stage III/IV chronic kidney disease, high-quality imaging may be obtained relatively safely using reduced volumes of isoosmolar, nonionic contrast with multidetector (32, 64, 128, or 220) scanners, particularly following preprocedural intravenous hydration. The CT dataset is rendered into threedimensional (3-D) images for dimensional postprocessing, a critical requirement for complex endovascular case planning.
Conventional arteriography adds little to the identification and analysis of iliac aneurysms; penetrating ulcers may appear like saccular aneurysms, and large aneurysms with circumferential mural thrombus may appear to have a normal contour.
SURGICAL MANAGEMENT
In general, iliac aneurysms are repaired when they reach 30 mm in diameter, become symptomatic, or rupture.
Due to the relatively inaccessible location of iliac aneurysms, situated deep in the pelvis, as well as densely adherent pelvic veins posterior to the arteries and frequent co-occurrence of calcific occlusive disease, conventional surgical repair is challenging and fraught with risk of significant hemorrhage. Thus, evolving endovascular methods of repair have largely supplanted open surgical reconstruction.
A variety of off-label devices and hybrid techniques have been applied to iliac aneurysm management. The variability derives, in large part, from uncertainty regarding the need to preserve antegrade internal iliac artery flow in most patients. Indications for internal iliac preservation remain controversial due to the added complexity, cost, and uncertain benefit derived from such procedures; analysis of the relative merits of intentional unilateral occlusion versus preservation in the management of iliac aneurysm disease is beyond the scope of this chapter.
Preoperative Planning
As in all things endovascular, high-quality imaging is critical for precase planning and, as previously mentioned, CT arteriography is optimal for this purpose. Using a combination of axial imaging and 3-D postprocessing, complete evaluation should, note the following:
Locations, diameter, and length of proximal and distal landing zones
Iliac artery tortuosity and angulation
Presence and severity of associated occlusive disease
Ipsilateral and contralateral internal iliac artery patency
Status of the ipsilateral deep femoral artery
Concomitant abdominal or thoracic aortic pathology
In general, landing zones are sited in nonaneurysmal arterial segments, manifesting minimal occlusive disease, with relative absence of angulation or tortuosity. The allowable diameter range for treatment may vary, depending on the particular device to be deployed. In all circumstances, reference should be made to the “Instructions for Use” included in the package insert.
Device selection is based on the need for durable aneurysm exclusion and endograft fixation, accomplished with the fewest component pieces possible.
This chapter focuses on endovascular and hybrid management strategies for the iliac bifurcation in the context of large common or internal iliac aneurysms. Standard techniques suffice for management of smaller (<24 mm) aneurysms that do
not involve the bifurcation, either in isolation or associated with larger proximal aortic aneurysms.
Positioning
Nearly all endovascular aortoiliac aneurysm repairs are performed with the patient in the supine position, with both arms tucked. The operative team stands on the patient’s right, with the C-arm brought in from the left. Although some operators prefer to access the left groin from the left side of the table, for a right-handed operator, it is ergonomically more natural to access both groins from the right.
Electrocardiogram (EKG) leads and other monitoring cables and lines are positioned so that they are not in the x-ray beam and do not entangle the C-arm gantry.
The left arm should be available for brachial artery access when necessary; it is not routinely prepped into the surgical field.
TECHNIQUES
ENDOVASCULAR COMMON ILIAC ARTERY ANEURYSM REPAIR WITH INTERNAL ILIAC ARTERY OCCLUSION
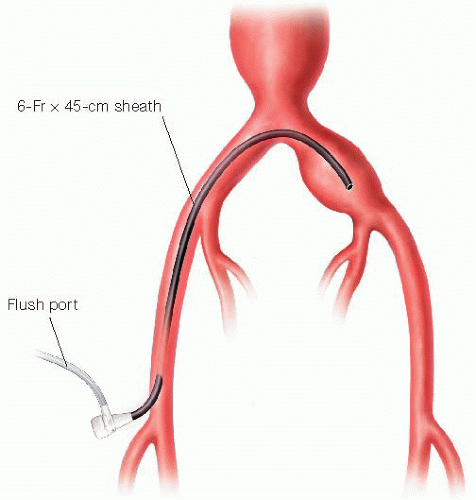
After obtaining bilateral femoral access, a shepherd’s hook-type (e.g., Omni™ Flush) side-hole catheter is advanced from the contralateral side for pelvic arteriography. Typical injection technique is 10 to 15 mL contrast at 15 mL per second. Following satisfactory anatomic delineation, a steerable hydrophilic 0.035 guidewire (e.g., angled Glidewire®, Terumo) is advanced through the same catheter into the ipsilateral external iliac artery. Following exchange for a stiffer guidewire (e.g., Rosen®), the flush catheter and contralateral femoral sheath are removed and replaced with a 45-cm 6-French (Fr) guide sheath (e.g., Balkan® or similar braided sheath), positioning the tip in the distal third of the common iliac aneurysm (FIG 1).
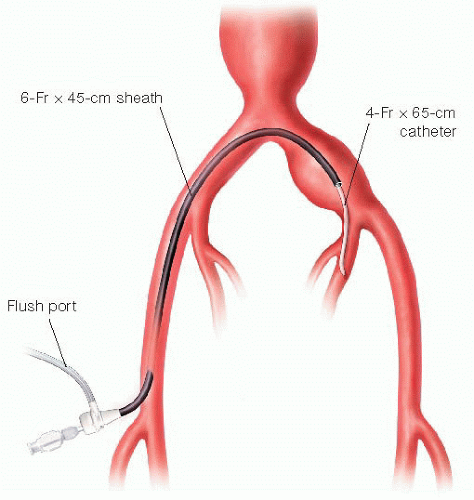
A 65-cm 4-Fr angled catheter (e.g., Kumpe®) is advanced through the sheath and, under digital subtraction roadmapping guidance, directs the hydrophilic guidewire into the ipsilateral internal iliac artery (FIG 2). The catheter is then advanced securely into either the anterior or posterior divisions. The C-arm gantry is positioned at approximately 30 degrees contralateral anterior-oblique for optimal visualization during this maneuver. With access secured, the 6-Fr sheath is then advanced over the 4-Fr catheter into the proximal internal iliac artery.
A selective internal iliac arteriogram is obtained through the 6-Fr sheath, and the angle catheter is retracted to the first bifurcation of the internal iliac artery. Typically, distal access is maintained with the wire during this maneuver.
[Alternate technique] Depending on anatomic considerations (e.g., tortuosity, iliac bifurcation angle, internal iliac branch anatomy, etc.), ipsilateral access may be
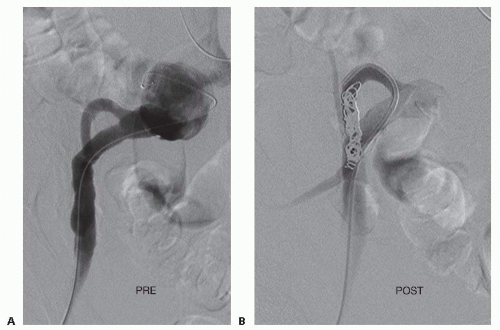
feasible. In this case, a 25-cm, 6-Fr sheath is advanced from the ipsilateral femoral artery. A 4- or 5-Fr appropriately shaped catheter (curve or reverse curve) directs the hydrophilic guidewire into the internal iliac artery (FIG 3). If a shepherd’s hook catheter type is employed, the hook may be reformed either in the iliac aneurysm or in the aorta, depending on their respective luminal diameters. The ipsilateral approach is more demanding technically and is not recommended when concomitant internal iliac aneurysm embolization is also indicated.
Size-appropriate platinum occlusion coils are deployed through the 4-Fr catheter into the main internal iliac artery trunk, with care taken to avoid placement or distal migration into arborizing branches or reflux back into the main iliac circulation. Alternative vascular occlusion devices may also be used for internal iliac embolization, including hydrogel coils and nitinol mesh (e.g., Amplatzer®) plugs. Interval arteriograms are obtained using small volume hand injections through the sheath or catheter to guide deployment and confirm positioning. Complete occlusion of the target artery during deployment is not necessary or even desirable. Following subsequent endograft deployment over the internal iliac artery orifice, in the absence of antegrade flow, the extensive surface area of the coils induces rapid thrombosis following reversal of anticoagulation at the end of the procedure. Usually less than five coils will suffice to ultimately induce complete occlusion.
[Alternate technique] The presence of an internal iliac aneurysm, either with or without an associated proximal common iliac aneurysm, deserves special consideration. In these situations, more extensive embolization of the internal iliac circulation will be necessary to prevent retrograde (type II) endoleak. Individual internal iliac branches must be separately embolized at the initial procedure (FIG 4).
If left untreated, due to collateral pelvic flow, these branches unfortunately remain patent following endograft deployment, severely limiting options for secondary procedures. Complete branch vessel embolization can be time consuming and tedious, due to the sheer number, anatomy and sizes of the branches that must be occluded, requiring patience, expert catheter and guidewire skills, and excellent intraoperative imaging.
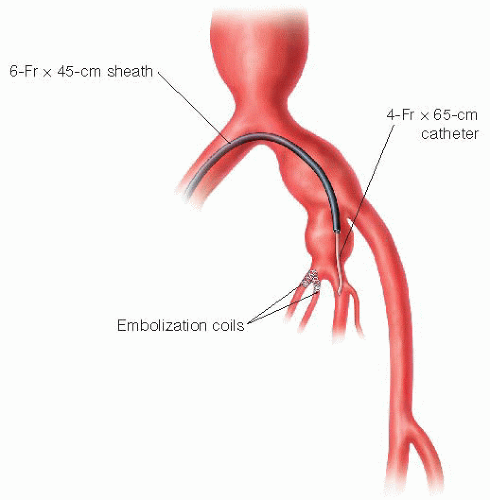
FIG 4 • In the setting of ipsilateral concomitant internal iliac aneurysm treatment, the contralateral approach should be used, and guide sheath advanced well within the internal iliac artery itself. Individual branches arising from the internal iliac aneurysm may prove difficult to identify and catheterize. Multiple projections of the C-arm gantry, both in lateral oblique and craniocaudal directions, should be employed to visualize the branches.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access