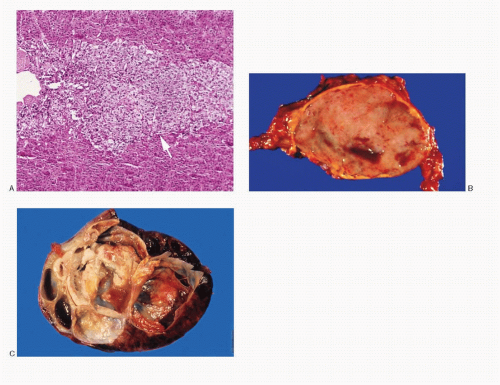
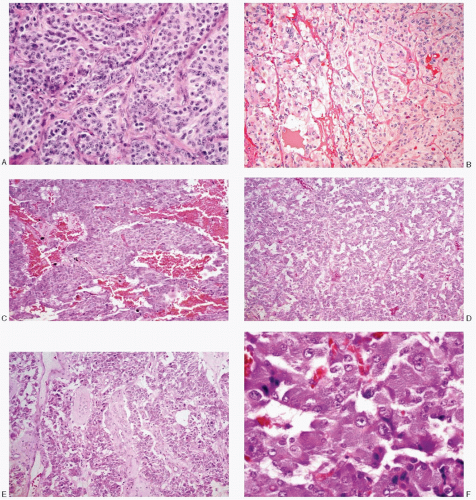
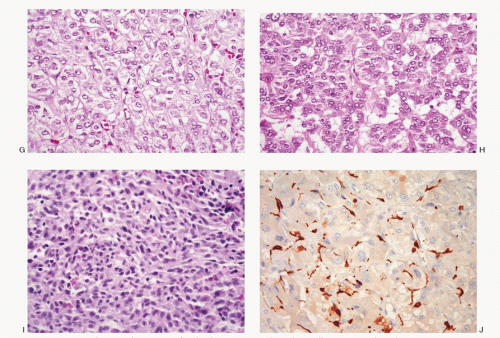
The specimens for cytologic diagnosis almost always represent fine needle aspiration (FNA) biopsies. The fine needle biopsy procedure is usually not recommended when pheochromocytoma is clinically suspected due to possible complications during the procedure. The documentation of cytologic features in literature is rather lean. The cytologic findings presented in this chapter represent both, the scrape preparations of the surgically excised neoplasms and FNA biopsies.
The cytologic features of pheochromocytomas vary widely from case to case and are dependent on the growth pattern, cell composition, as well as the cell characteristics (
Table 14.1;
Figs. 14.3,
14.4,
14.5,
14.6,
14.7,
14.8,
14.9,
14.10,
14.11,
14.12 and
14.13). The aspirates tend to be bloody. The cellularity of the aspirate is variable and often compromised due to excessive blood. The neoplastic cells appear isolated, in loosely cohesive groups and in syncytial tissue fragments, without any architectural patterns. The neoplastic cells vary in size and range from small to large with frequent giant forms. The cells in a given case can be monomorphic or pleomorphic. They can be round, cuboidal, plasmacytoid, polygonal, racquet shaped, caudate, or spindle shaped. The characteristic large polygonal cells with deep eosinophilic cytoplasm, seen in histologic preparations, are only rarely appreciated in cytologic smears. The cell borders are well to poorly defined. Their nuclei are central to eccentric, round to ovoid with stippled chromatin (salt-pepper) and small nucleoli. The nuclear pleomorphism may be striking with the presence
of giant forms with pyknosis (
Figs. 14.6B,C and
14.8C). Intranuclear inclusions may also be present. Their cytoplasm of pheochromocytoma cells is variable. The nuclear/cytoplasmic ratios likewise vary. Some cells appear large and ganglion-like (
Fig. 14.8C), while some exhibit wispy cytoplasmic processes (
Fig. 14.8B). It is not unusual to encounter disruption of the lipid-rich cytoplasm resulting in a large population of uniform naked nuclei (
Figs. 14.12 and
14.13A). The cytoplasm of the pheochromocytoma cells may contain eosinophilic, periodic acid-Schiff (PAS)-positive globules. Red cytoplasmic granules are seen in Romanowsky-stained preparations. Old and new hemorrhage and degenerative changes are frequent.