Objectives
- Identify the functional anatomy and zones of the adrenal glands and the principal hormones secreted from each zone.
- Describe and contrast the regulation of synthesis and release of the adrenal steroid hormones (glucocorticoids, mineralocorticoids, and androgens) and the consequences of abnormalities in their biosynthetic pathways.
- Understand the cellular mechanism of action of adrenal cortical hormones and identify their major physiologic actions, particularly during injury and stress.
- Identify the major mineralocorticoids, their biologic actions, and their target organs or tissues.
- Describe the regulation of mineralocorticoid secretion and relate this to the regulation of sodium and potassium excretion.
- Identify the causes and consequences of oversecretion and undersecretion of glucocorticoids, mineralocorticoids, and adrenal androgens.
- Identify the chemical nature of catecholamines and their biosynthesis and metabolic fate.
- Describe the biologic consequences of sympatho-adrenal medulla activation and identify the target organs or tissues for catecholamine effects along with the receptor types that mediate their actions.
- Describe and integrate the interactions of adrenal medullary and cortical hormones in response to stress.
- Identify diseases caused by oversecretion of adrenal catecholamines.
Adrenal Gland: Introduction
The adrenal glands are important components of the endocrine system. They contribute significantly to maintaining homeostasis particularly through their role in the regulation of the body’s adaptive response to stress, in the maintenance of body water, sodium and potassium balance, and in the control of blood pressure. The main hormones produced by the human adrenal glands belong to 2 different families based on their structure; these are the steroid hormones including the glucocorticoids, mineralocorticoids and androgens; and the catecholamines norepinephrine and epinephrine. The adrenal gland, like the pituitary, has 2 different embryologic origins, which as we will discuss, influence the mechanisms that control hormone production by each of the 2 components.
Functional Anatomy and Zonation
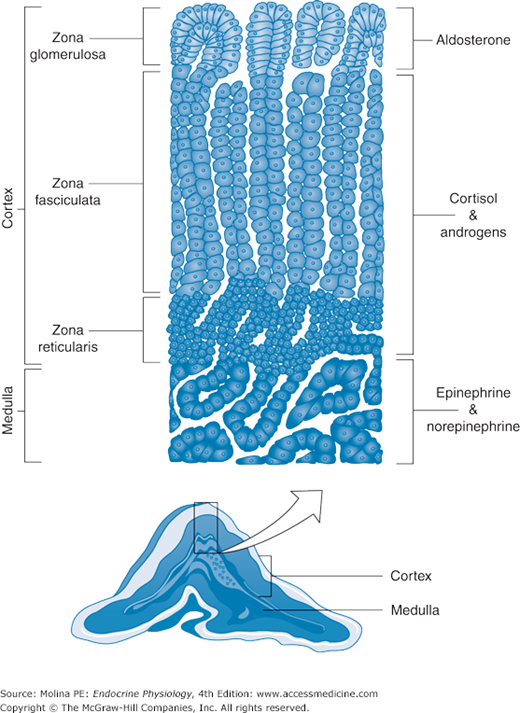
The adrenal glands are located above the kidneys. They are small, averaging 3–5 cm in length, and weigh 1.5–2.5 g and as mentioned above, consist of 2 different components; the cortex and the medulla (Figure 6–1), each with a specific embryologic origin. The outer adrenal cortex is derived from mesodermal tissue and accounts for approximately 90% of the weight of the adrenals. The cortex synthesizes the adrenal steroid hormones called glucocorticoids, mineralocorticoids, and androgens (eg, cortisol, aldosterone, and dehydroepiandrosterone [DHEA]) in response to hypothalamic-pituitary-adrenal hormone stimulation (Figure 6–2). The inner medulla is derived from a subpopulation of neural crest cells and makes up the remaining 10% of the mass of the adrenals. The medulla synthesizes catecholamines (eg, epinephrine and norepinephrine) in response to direct sympathetic (sympatho-adrenal) stimulation.
Figure 6–1.
Adrenal glands. The adrenal glands are composed of a cortex and a medulla, each derived from a different embryologic origin. The cortex is divided into 3 zones: reticularis, fasciculata, and glomerulosa. The cells that make up the 3 zones have distinct enzymatic capacities, leading to a relative specificity in the products of each of the adrenal cortex zones. The adrenal medulla is made of cells derived from the neural crest.
Figure 6–2.
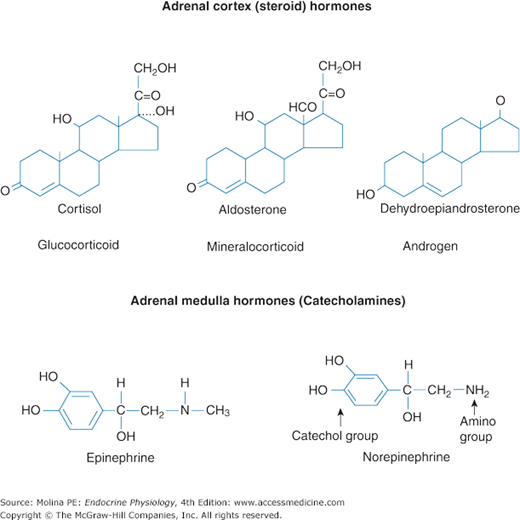
Adrenal gland hormones. The principal hormones synthesized and released by the adrenal cortex are the glucocorticoid cortisol, the mineralocorticoid aldosterone, and the androgen dehydroepiandrosterone (DHEA). These steroid hormones are derived from cholesterol. The principal hormones synthesized and released by the adrenal medulla are the catecholamines epinephrine and norepinephrine. These catecholamines are derived from L-tyrosine.
Several features of the adrenal glands contribute to the regulation of steroid hormone and catecholamine synthesis, including the architecture, blood supply, and the enzymatic machinery of the individual cells. Blood supply to the adrenal glands is derived from the superior, middle, and inferior suprarenal arteries. Branches of these arteries form a capillary network arranged so that blood flows from the outer cortex toward the center area, following a radially oriented sinusoid system. This direction of blood flow controls the access of steroid hormones to the circulation and concentrates the steroid hormones at the core of the adrenals, thus modulating the activities of enzymes involved in catecholamine synthesis. The venous drainage of the adrenal glands involves a single renal vein on each side; the right vein drains into the inferior vena cava and the left vein drains into the left renal artery.
Hormones of the Adrenal Cortex
The adrenal cortex consists of 3 zones that vary in both their morphologic and functional features and thus, the steroid hormones they produce (see Figure 6–1).
- The zona glomerulosa contains abundant smooth endoplasmic reticulum and is the unique source of the mineralocorticoid aldosterone.
- The zona fasciculata contains abundant lipid droplets and produces the glucocorticoids, cortisol and corticosterone, and the androgens, DHEA and DHEA sulfate (DHEAS).
- The zona reticularis develops postnatally and is recognizable at approximately age 3 years; it also produces glucocorticoids and androgens.
The products of the adrenal cortex are classified into 3 general categories: glucocorticoids, mineralocorticoids, and androgens (see Figure 6–2) which reflect the primary effects mediated by these hormones. This will become clear when their individual target organ effects are discussed.
Steroid hormones share an initial step in their biosynthesis (steroidogenesis), which is the conversion of cholesterol to pregnenolone (Figure 6–3). Cholesterol used for steroid hormone synthesis can be derived from the plasma membrane or from the steroidogenic cytoplasmic pool of cholesteryl-esters. Free cholesterol is generated by the action of the enzyme cholesterol ester hydrolase. Cholesterol is transported from the outer mitochondrial membrane to the inner mitochondrial membrane, followed by the conversion to pregnenolone by P450scc enzyme; an inner mitochondrial membrane present in all steroidogenic cells. This is considered the rate-limiting step in steroid hormone synthesis and requires the STeroid Acute Regulatory (STAR) protein. STAR is critical in mediating cholesterol transfer to the inner mitochondrial membrane and the cholesterol side chain cleavage enzyme system.
Figure 6–3.
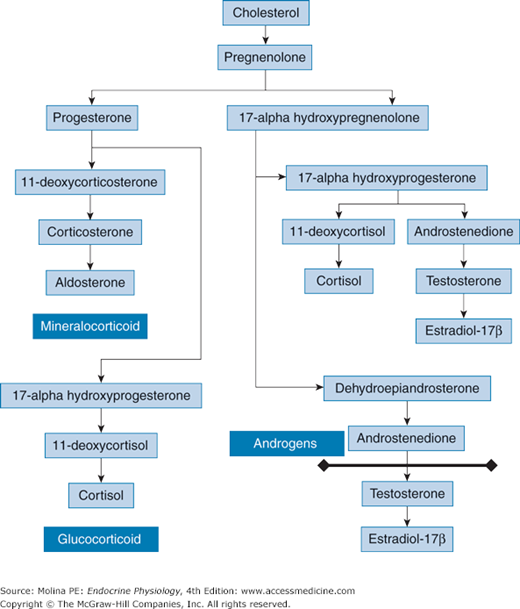
Adrenal steroid hormone synthetic pathway. Cholesterol is converted to pregnenolone by the cytochrome P450 side-chain cleavage enzyme. Pregnenolone is converted to progesterone by 3β-hydroxysteroid dehydrogenase or to 17α-OH-pregnenolone by 17α-hydroxylase. Thereafter, 17α-OH-pregnenolone is converted to 17α-OH-progesterone by 3β-hydroxysteroid dehydrogenase, 17α-OH-progesterone is converted to 11-deoxycortisol by the enzyme 21-hydroxylase, and 11-deoxycortisol is converted to cortisol by 11β-hydroxylase. In addition, 17α-OH-progesterone can be converted to androstenedione. Both 17α-OH-pregnenolone and 17α-OH-progesterone can be converted to the androgens dehydroepiandrosterone (DHEA) and androstenedione, respectively. DHEA is converted to androstenedione. Cells in the zona glomerulosa do not have 17α-hydroxylase activity. Therefore, pregnenolone can be converted only into progesterone. The zona glomerulosa possesses aldosterone synthase activity, and this enzyme converts deoxycorticosterone to corticosterone, corticosterone to 18-hydroxycorticosterone, and 18-hydroxycorticosterone to aldosterone, the principal mineralocorticoid produced by the adrenal glands. The line denotes which steps occur outside the adrenal glands.
This conversion of cholesterol to pregnenolone is the first step in a sequence of multiple enzymatic reactions involved in the synthesis of steroid hormones. Because the cells that constitute the different sections of the adrenal cortex have specific enzymatic features, the synthetic pathway of steroid hormones will result in preferential synthesis of glucocorticoids, mineralocorticoids, or androgens, depending on the region.
Cells of the adrenal zona fasciculata and zona reticularis synthesize and secrete the glucocorticoids cortisol or corticosterone through the following pathway (see Figure 6–3). Pregnenolone exits the mitochondria and is converted to either progesterone or 17α-OH-pregnenolone. Conversion of pregnenolone to progesterone is mediated by 3β-hydroxysteroid dehydrogenase. Progesterone is converted to 11-deoxycorticosterone by 21-hydroxylase; then 11-deoxycorticosterone is converted to corticosterone by 11β-hydroxylase. Conversion of pregnenolone to 17α-OH-pregnenolone is mediated by 17α-hydroxylase; 17α-OH-pregnenolone is converted to 17α-OH-progesterone by 3β-hydroxysteroid dehydrogenase; 17α-OH-progesterone is converted to either 11-deoxycortisol or androstenedione. The enzyme 21-hydroxylase mediates the conversion of 17α-OH-progesterone to 11-deoxycortisol, which is then converted to cortisol by 11β-hydroxylase. Both 17α-OH-pregnenolone and 17α-OH-progesterone can be converted to the androgens DHEA and androstenedione, respectively. DHEA is converted to androstenedione by 3β-hydroxysteroid dehydrogenase.
The adrenal zona glomerulosa cells preferentially synthesize and secrete the mineralocorticoid aldosterone. The cells of the zona glomerulosa do not have 17α-hydroxylase activity. Therefore, pregnenolone can be converted only to progesterone. The zona glomerulosa possesses aldosterone synthase activity, and this enzyme converts 11-deoxycorticosterone to corticosterone, corticosterone to 18-hydroxycorticosterone, and 18-hydroxycorticosterone to aldosterone, the principal mineralocorticoid produced by the adrenal glands.
The initial steps in the biosynthesis of DHEA from cholesterol are similar to those involved in glucocorticoid and mineralocorticoid hormone synthesis. The product of these initial enzymatic conversions, pregnenolone, undergoes 17α-hydroxylation by microsomal P450c17 and conversion to DHEA. 17α-pregnenolone can also be converted to 17α-OH-progesterone, which in turn can be converted to androstenedione in the zona fasciculata.
As already mentioned, the initial steps in the biosynthetic pathways of steroid hormones are identical regardless of the steroid hormone synthesized. The production of the hormones can be regulated acutely and chronically. Acute regulation results in the rapid production of steroids in response to immediate need and occurs within minutes of the stimulus. The biosynthesis of glucocorticoids to combat stressful situations and the rapid synthesis of aldosterone to rapidly regulate blood pressure are examples of this type of regulation. Chronic stimulation, such as that which occurs during prolonged starvation and chronic disease, involves the synthesis of enzymes involved in steroidogenesis to enhance the synthetic capacity of the cells. Although both glucocorticoids and mineralocorticoids are released in response to stressful conditions, the conditions under which they are stimulated differ, and the cellular mechanisms responsible for stimulating their release are different. Thus, the mechanisms involved in the regulation of their release differ and are specifically controlled as described below.
![]() The pulsatile release of cortisol is under direct stimulation by adrenocorticotropic hormone (ACTH) released from the anterior pituitary. ACTH, or corticotropin, is synthesized in the anterior pituitary as a large precursor, proopiomelanocortin (POMC). POMC is processed post-translationally into several peptides, including corticotropin, β-lipotropin, and β-endorphin, as presented and discussed in Chapter 3 (see Figure 3–4). The release of ACTH is pulsatile with approximately 7–15 episodes per day. The stimulation of cortisol release occurs within 15 minutes of the surge in ACTH. An important feature in the release of cortisol is that in addition to being pulsatile, it follows a circadian rhythm that is exquisitely sensitive to environmental and internal factors such as light, sleep, stress, and disease (see Figure 1–8). Release of cortisol is greatest during the early waking hours, with levels declining as the afternoon progresses. As a result of its pulsatile release, the resulting circulating levels of the hormone vary throughout the day, and this has a direct impact on how cortisol values are interpreted according to the timing of blood sample collection.
The pulsatile release of cortisol is under direct stimulation by adrenocorticotropic hormone (ACTH) released from the anterior pituitary. ACTH, or corticotropin, is synthesized in the anterior pituitary as a large precursor, proopiomelanocortin (POMC). POMC is processed post-translationally into several peptides, including corticotropin, β-lipotropin, and β-endorphin, as presented and discussed in Chapter 3 (see Figure 3–4). The release of ACTH is pulsatile with approximately 7–15 episodes per day. The stimulation of cortisol release occurs within 15 minutes of the surge in ACTH. An important feature in the release of cortisol is that in addition to being pulsatile, it follows a circadian rhythm that is exquisitely sensitive to environmental and internal factors such as light, sleep, stress, and disease (see Figure 1–8). Release of cortisol is greatest during the early waking hours, with levels declining as the afternoon progresses. As a result of its pulsatile release, the resulting circulating levels of the hormone vary throughout the day, and this has a direct impact on how cortisol values are interpreted according to the timing of blood sample collection.
ACTH stimulates cortisol release by binding to a Gαs protein–coupled plasma membrane melanocortin 2 receptor on adrenocortical cells, resulting in activation of adenylate cyclase, an increase in cyclic adenosine monophosphate, and activation of protein kinase A (see Figure 3–4). Protein kinase A phosphorylates the enzyme cholesteryl-ester hydrolase, increasing its enzymatic activity; leading to increased cholesterol availability for hormone synthesis. In addition, ACTH activates and increases the synthesis of STAR, the enzyme involved in the transport of cholesterol into the inner mitochondrial membrane. Therefore, ACTH stimulates the 2 initial and rate-limiting steps in steroid hormone synthesis.
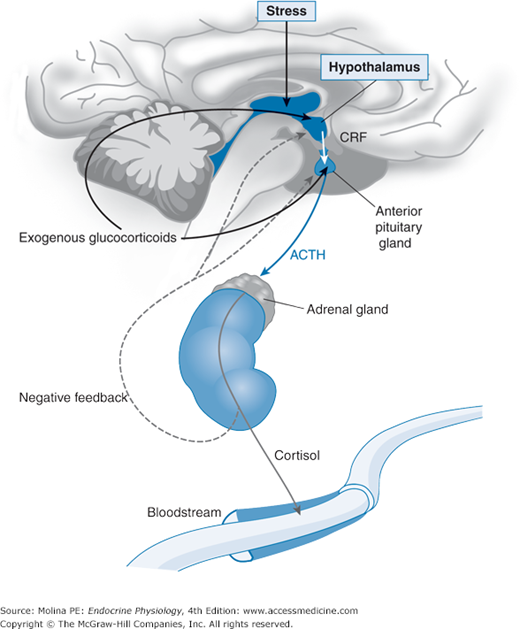
The release of ACTH from the anterior pituitary is regulated by the hypothalamic peptide corticotropin-releasing hormone (CRH) discussed in Chapter 3. Cortisol inhibits the biosynthesis and secretion of CRH and ACTH in a classic example of negative feedback regulation by hormones. This closely regulated circuit is referred to as the hypothalamic-pituitary-adrenal (HPA) axis (Figure 6–4).
Figure 6–4.
Hypothalamic-pituitary-adrenal axis. Corticotropin-releasing factor (CRF), produced by the hypothalamus and released in the median eminence, stimulates the synthesis and processing of proopiomelanocortin, with resulting release of proopiomelanocortin peptides that include adrenocorticotropic hormone (ACTH) from the anterior pituitary. ACTH binds to the melanocortin-2 receptor in the adrenal gland and stimulates the cholesterol-derived synthesis of adrenal steroid hormones. Glucocorticoids released into the systemic circulation exert negative feedback inhibition of corticotropin-releasing factor (CRF) and ACTH release from the hypothalamus and pituitary, respectively, in a classic example of negative feedback hormone regulation. This closely regulated circuit is referred to as the hypothalamic-pituitary-adrenal (HPA) axis.
Because of their lipophilic nature, free cortisol molecules are mostly insoluble in water. Therefore, cortisol is usually found in biologic fluids either in a conjugated form (eg, as sulfate or glucuronide derivatives) or bound to carrier proteins (noncovalent, reversible binding). The majority of cortisol is bound to glucocorticoid-binding α2-globulin (transcortin or cortisol-binding globulin [CBG]), a specific carrier of cortisol. Normal levels of CBG average 3–4 mg/dL and are saturated with cortisol levels of 28 μg/dL. The hepatic synthesis of CBG is stimulated by estrogen and decreased by hepatic disease (cirrhosis). Approximately 20%–50% of bound cortisol is bound nonspecifically to plasma albumin. A small amount (<10%) of total plasma cortisol circulates unbound and is referred to as the free fraction. This is considered to represent the biologically active fraction of the hormone that is directly available for action.
As discussed in Chapter 1, the major role of plasma-binding proteins is to act as a “buffer” or reservoir for active hormones. Protein-bound steroids are released into the plasma in free form as soon as the free hormone concentration decreases. Plasma-binding proteins also protect the hormone from peripheral metabolism (notably by liver enzymes) and increase the half-life of biologically active forms. The half-life of cortisol is 70–90 minutes.
Because of their lipophilic nature, steroid hormones diffuse easily through cell membranes and therefore have a large volume of distribution. In their target tissues, steroid hormones are concentrated by an uptake mechanism that relies on their binding to intracellular receptors.
The liver and kidney are the 2 major sites of hormone inactivation and elimination. Several pathways are involved in this process, including reduction, oxidation, hydroxylation, and conjugation, to form the sulfate and glucuronide derivatives of the steroid hormones. These processes occur in the liver through phase I and phase II biotransformation reactions, leading to generation of a more water-soluble compound for easier excretion. Inactivation of cortisol to cortisone and to tetrahydrocortisol and tetrahydrocortisone is followed by conjugation and renal excretion. These metabolites are referred to as 17-hydroxycorticosteroids, and their determination in 24-hour urine collections is used to assess the status of adrenal steroid production.
Localized tissue metabolism contributes to modulation of the physiologic effects of glucocorticoids by the isoforms of the enzyme 11β-hydroxysteroid dehydrogenase. Corticosteroid 11β-hydroxysteroid dehydrogenase type I is a low-affinity nicotinamide adenine dinucleotide phosphate–dependent reductase that converts cortisone back to its active form, cortisol. This enzyme is expressed in liver, adipose tissue, lung, skeletal muscle, vascular smooth muscle, gonads, and the central nervous system. The high expression of this enzyme, particularly in adipose tissue has gained recent attention, as it is thought to contribute to the pathophysiology of metabolic syndrome (see Chapter 10).
The conversion of cortisol to cortisone, its less active metabolite, is mediated by the enzyme 11β-hydroxysteroid dehydrogenase type II. This high-affinity nicotinamide adenine dinucleotide–dependent dehydrogenase is expressed primarily in the distal convoluted tubules and collecting ducts of the kidney, where it contributes to specificity of mineralocorticoid hormone effects. As discussed below, conversion of cortisol to cortisone is critical in preventing excess mineralocorticoid activity resulting from cortisol binding to the mineralocorticoid receptor. Increased expression and activity of 11β-hydroxysteroid dehydrogenase type I amplifies glucocorticoid action within the cell, whereas increased 11β-hydroxysteroid dehydrogenase type II activity decreases glucocorticoid action.
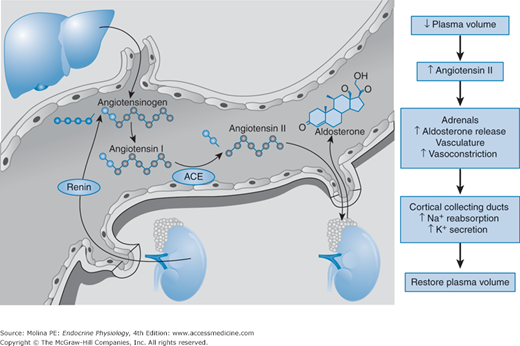
![]() Aldosterone synthesis and release in the adrenal zona glomerulosa is predominantly regulated by angiotensin II and extracellular K+ and, to a lesser extent, by ACTH. Aldosterone is part of the renin-angiotensin-aldosterone system, which is responsible for preserving circulatory homeostasis in response to a loss of salt and water (eg, with intense and prolonged sweating, vomiting, or diarrhea). The components of the renin-angiotensin-aldosterone system respond quickly to reductions in intravascular volume and renal perfusion. Angiotensin II is the principal stimulator of aldosterone production when intravascular volume is reduced.
Aldosterone synthesis and release in the adrenal zona glomerulosa is predominantly regulated by angiotensin II and extracellular K+ and, to a lesser extent, by ACTH. Aldosterone is part of the renin-angiotensin-aldosterone system, which is responsible for preserving circulatory homeostasis in response to a loss of salt and water (eg, with intense and prolonged sweating, vomiting, or diarrhea). The components of the renin-angiotensin-aldosterone system respond quickly to reductions in intravascular volume and renal perfusion. Angiotensin II is the principal stimulator of aldosterone production when intravascular volume is reduced.
Both angiotensin II and K+ stimulate aldosterone release by increasing intracellular Ca2+ concentrations. Angiotensin II receptor-mediated signaling leads to increased intracellular calcium levels, while increased K+ concentrations depolarize the cell leading to Ca2+ influx via voltage-gated L- and T-type Ca2+ channels.
The main physiologic stimulus for aldosterone release is a decrease in the effective intravascular blood volume (Figure 6–5). A decline in blood volume leads to decreased renal perfusion pressure, which is sensed by the juxtaglomerular apparatus (baroreceptor) and triggers the release of renin. Renin release is also regulated by sodium chloride (NaCl) concentration in the macula densa, plasma electrolyte concentrations, angiotensin II levels, and sympathetic tone. Renin catalyzes the conversion of angiotensinogen, a liver-derived protein, to angiotensin I. Circulating angiotensin I is converted to angiotensin II by angiotensin-converting enzyme (ACE), highly expressed in vascular endothelial cells. The increase in circulating angiotensin II produces direct arteriolar vasoconstriction, stimulates adrenocortical cells of the zona glomerulosa to synthesize and release aldosterone, and stimulates arginine vasopressin release from the posterior pituitary (see Chapter 2).
Figure 6–5.
Regulation of aldosterone release by the renin-angiotensin-aldosterone system. A decrease in the effective circulating blood volume triggers the release of renin from the juxtaglomerular apparatus in the kidney. Renin cleaves angiotensinogen, the hepatic precursor of angiotensin peptides, to form angiotensin I. Angiotensin I is converted to angiotensin II by angiotensin-converting enzyme (ACE), which is bound to the membrane of vascular endothelial cells. Angiotensin II is a potent vasoconstrictor and stimulates the production of aldosterone in the zona glomerulosa of the adrenal cortex. Aldosterone production is also stimulated by potassium, ACTH, norepinephrine, and endothelins. (Modified, with permission, from Weber KT. Mechanisms of disease: aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689. Copyright © Massachusetts Medical Society. All rights reserved.)
Potassium is also a major physiologic stimulus for aldosterone production, illustrating a classic example of hormone regulation by the ion it controls. Aldosterone is critical in maintaining potassium homeostasis by increasing K+ excretion in urine, feces, sweat, and saliva, preventing hyperkalemia during periods of high K+ intake or after K+ release from skeletal muscle during strenuous exercise. In turn, elevations in circulating K+ concentrations stimulate the release of aldosterone from the adrenal cortex.
The total amount of aldosterone released is markedly less than that of glucocorticoids. Plasma aldosterone levels average 0.006–0.010 μg/dL (in contrast to cortisol levels of 13.5 μg/dL). Secretion can be increased 2- to 6-fold by sodium depletion or by a decrease in the effective circulating blood volume, such as occurs with ascites. Binding of aldosterone to plasma proteins is minimal, resulting in a short plasma half-life of approximately 15–20 minutes. This fact is relevant to mineralocorticoid and glucocorticoid receptor-mediated effects, and their specificity as will be discussed below.
Aldosterone is metabolized in the liver to tetrahydroglucuronide derivative and excreted in the urine. A fraction of aldosterone is metabolized to aldosterone 18-glucuronide, which can be hydrolyzed back to free aldosterone under low pH conditions; thus it is an “acid-labile conjugate.” Approximately 5% of aldosterone is excreted in the acid-labile form; a small fraction of aldosterone appears intact in the urine (1%) and up to 40% is excreted as tetraglucuronide.
The third class of steroid hormones produced by the zona reticularis of the adrenal glands is the adrenal androgens, including DHEA and DHEAS (see Figure 6–3). DHEA is the most abundant circulating hormone in the body and is readily conjugated to its sulfate ester DHEAS. Its production is controlled by ACTH.
The adrenal androgens are converted into androstenedione and then into potent androgens or estrogens in peripheral tissues. The synthesis of dihydrotestosterone and 17β-estradiol, the most potent androgen and estrogen from DHEA, respectively, involves several enzymes, including 3β-hydroxysteroid dehydrogenase/D5-D4 isomerase, 17β-hydroxysteroid dehydrogenase, and 5β-reductase or aromatase (see Chapters 8 and 9). The importance of the adrenal-derived androgens to the overall production of sex steroid hormones is highlighted by the fact that approximately 50% of total androgens in the prostate of adult men are derived from adrenal steroid precursors.
The control and regulation of the release of adrenal sex steroids are not completely understood. However, it is known that adrenal secretion of DHEA and DHEAS increases in children at the age of 6–8 years, and values of circulating DHEAS peak between the ages of 20 and 30 years. Thereafter, serum levels of DHEA and DHEAS decrease markedly. In fact, at 70 years of age, serum DHEAS levels are at approximately 20% of their peak values and continue to decrease with age. This 70%–95% reduction in the formation of DHEAS by the adrenal glands during the aging process results in a dramatic reduction in the formation of androgens and estrogens in peripheral target tissues. Despite the marked decrease in the release of DHEA as the individual ages, this is not paralleled by a similar decrease in ACTH or cortisol release. The clinical impact of this age-related deficiency in DHEA production is not fully understood but may play an important role in the regulation of immune function and intermediary metabolism, among other aspects of physiology of the aging process.
![]() The physiologic effects of steroid hormones can be divided into genomic and nongenomic effects. Most of the physiologic effects of glucocorticoid and mineralocorticoid hormones are mediated through binding to intracellular receptors that operate as ligand-activated transcription factors to regulate gene expression. Binding of steroid hormones to their specific receptors leads to conformational changes in the receptor, leading to their ability to act as a ligand-dependent transcription factors. The steroid-receptor complex binds to hormone-responsive elements on the chromatin and thereby regulates gene transcription, resulting in the synthesis or repression of proteins, which are ultimately responsible for the physiologic effects of the hormones.
The physiologic effects of steroid hormones can be divided into genomic and nongenomic effects. Most of the physiologic effects of glucocorticoid and mineralocorticoid hormones are mediated through binding to intracellular receptors that operate as ligand-activated transcription factors to regulate gene expression. Binding of steroid hormones to their specific receptors leads to conformational changes in the receptor, leading to their ability to act as a ligand-dependent transcription factors. The steroid-receptor complex binds to hormone-responsive elements on the chromatin and thereby regulates gene transcription, resulting in the synthesis or repression of proteins, which are ultimately responsible for the physiologic effects of the hormones.
Steroid hormones can also exert their physiologic effects through nongenomic actions. A nongenomic action is any mechanism that does not directly involve gene transcription, such as the rapid steroid effects on the electrical activity of nerve cells or the interaction of steroid hormones with the receptor for γ-aminobutyric acid. In contrast to the genomic effects, nongenomic effects require the continued presence of the hormone and occur more quickly because they do not require the synthesis of proteins. Some of the nongenomic effects may be mediated by specific receptors located on the cell membrane. The nature of these receptors and the signal transduction mechanisms involved are not completely understood and are still under investigation.