Diagnose a mass in adrenal or at another site of paraganglia
Evaluate mass for malignancy
Intraoperative diagnosis may not be needed to guide surgical management in many cases
Tissue may be taken for ancillary studies for some tumors
Additional surgery may be performed if malignancy is diagnosed
Adrenal lesions may be detected due to functional tumors causing clinical syndromes or as image-detected masses
Majority of tumors are cortical adenomas
˜ 15% are detected due to clinical syndromes
Cushing syndrome: Excess cortisol
Conn syndrome: Excess aldosterone
Virilization or feminization: Excess sex steroids
Many nonfunctional tumors are found as “incidentalomas” on imaging performed for unrelated symptoms
Pheochromocytomas are usually detected by clinical symptoms
Paroxysmal hypertension, tachycardia, diaphoresis, and headache
Diagnosis confirmed by plasma or urine tests for catecholamines and metanephrines
Adrenal gland may be removed as part of a radical nephrectomy for renal cell carcinoma
Incidental adrenal lesions are usually small adenomas
Metastatic renal cell carcinoma to adrenal is less common
Bilateral gland involvement can be due to adrenal cortical hyperplasia, hereditary pheochromocytoma, or metastases
Tumors arise less commonly from other paraganglia
Complete excision
Ink outer surface
Serially section through gland at 3-mm intervals
Identify all masses present
Size
Number
Location: Arising in cortex or medulla or extraadrenal with secondary adrenal involvement
Border: Circumscribed or infiltrative
Color
Necrosis
Evaluate adjacent adrenal tissue
Normal: Golden yellow cortex ˜ 3 mm, central pearly gray medulla
Cortical hyperplasia: Diffuse or nodular enlargement of cortex
Cortical atrophy: Cortex < 2 mm in thickness, fibrous thickening of capsule
Medullary hyperplasia: Diffuse or nodular enlargement of medulla
Assess involvement of adjacent tissues or organs if present
Needle biopsy
Biopsies may be submitted to determine if adequate tissue for diagnosis is present
Small representative section of lesion may be frozen
Only lesions > 1 cm in size should be examined by frozen section
Entire lesion should never be frozen
Cytological examination may be very helpful for diagnosis
Origin of adrenal tumors (cortical or medullary)
Diagnosis of metastatic tumors
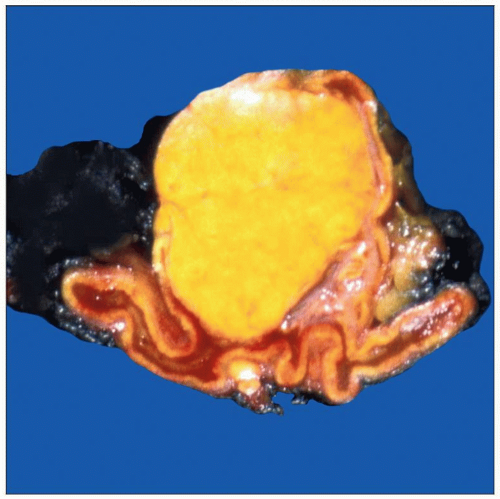
Well-circumscribed mass arising from cortex
Usually unilateral and solitary
Majority < 5 cm
Carcinomas are usually larger
Tumor cells arranged in nesting/alveolar pattern, short cords, anastomosing trabeculae, or mixture of patterns
Mitotic figures absent or rare
Necrosis uncommon
Cushing syndrome
Moderately sized adenomas with bright yellow color
Cause suppression of ACTH by producing cortisol
Results in atrophy of normal gland
Conn syndrome
Often small (< 2 cm) and pale in color
Overproduce aldosterone
Normal gland is not affected
Adenomas associated with virilization or feminization
Typically large (> 10 cm) and tan-white to brown
Nonfunctioning adenomas
May be small or large
Geographic or mottled zones of dark pigmentation may be present
Bulky tumors with red-brown fleshy, firm appearance
Typically unilateral and large
If bilateral, consider contralateral metastasis
It is not possible to predict malignant behavior with certainty
Paraganglia are distributed symmetrically from base of skull to pelvis
Most common site for neoplasms is adrenal medulla
Other sites include
Carotid body (at bifurcation of carotid artery)
Glomus tympanicum (middle ear)
Glomus jugulare (jugular foramen)
Organ of Zuckerkandl (at bifurcation of aorta or origin of inferior mesenteric artery)
Increased malignant potential is observed for head and neck sites
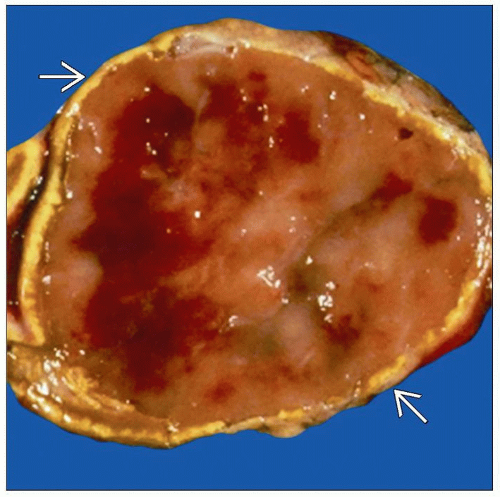
Typically yellow-white to red-brown circumscribed tumors 5-8 cm in size
May have necrosis, hemorrhage, or cystic degeneration
˜ 10% are bilateral
In adrenal, these tumors arise from medulla
˜ 30% are associated with hereditary syndromes
At least 10 susceptibility genes have been identified
Medullary hyperplasia may be present (increased thickness &/or multiple nodules)
Cells have a nested zellballen pattern
Basophilic cytoplasm; bizarre, isolated, atypical nuclei
Zellballen are surrounded by inconspicuous glial-type sustentacular cells
˜ 10% will have malignant behavior (locally invasive with metastases)
Difficult to predict this group based on histologic features
Features associated with, but not diagnostic of, malignant behavior include
Large nests or diffuse growth
Central or confluent tumor necrosis
High cellularity
Spindle cell pattern
High mitotic rate (> 3 mitoses/10 HPF)
Vascular or capsular invasion
Majority of metastatic carcinomas are from lung or kidney
More likely to be bilateral
May be difficult to determine origin of primary tumor
Well-circumscribed, soft tan-yellow to focally red-brown mass
Resembles adipose tissue with focal fibrous areas
Lesion is within adrenal gland and may compress it
Tumor consists of adipose tissue and bone marrow elements
20% associated with tuberous sclerosis
Diffuse: Uniform increase in thickness of cortex
Most commonly due to pituitary Cushing disease (pituitary adenoma producing ACTH)
Nodular: Multiple nodules in both glands
Most commonly primary hyperplasia (etiology unknown)
Bilateral adrenal involvement with multiple pigmented (black, brown, or red) nodules of cortical hyperplasia
Clinical history of Cushing syndrome
90% of cases associated with Carney complex
Usually small and unilocular and filled with serous or serosanguineous fluid
May arise from blood vessels or lymphatics
Some are pseudocysts without identifiable lining
Identification of normal adrenal is crucial to confirm lesion did not arise from the adrenal
Lymphomas can arise from adjacent nodes and surround adrenal
Tissue for ancillary studies to identify tumor may be helpful
All very rare
More likely to be neuroblastoma, ganglioneuroblastoma, or ganglioneuroma than cortical tumors or pheochromocytoma
Neuroblastoma
Soft and hemorrhagic with frequent areas of necrosis
Cysts may be present
May invade into surrounding tissue
Ganglioneuroma and ganglioneuroblastoma
Firmer, white to tan, and may have areas of calcification
If there are gross areas resembling neuroblastoma, these should be sampled for ancillary testing
Eligibility for treatment protocols is often based on results of ancillary testing
Nonfixed tissue may be required for cytogenetic studies, molecular studies (frozen), and electron microscopy
Presence or absence of a neoplasm
Specific diagnosis when possible
If a definitive diagnosis of adenoma or carcinoma or pheochromocytoma can be made, this should be reported
Report if invasion into large vessels or adjacent structures is identified
There is no need to report margins
Report diagnosis when possible
Cytoplasm of adrenal adenoma cells is vacuolated whereas the cytoplasm of a renal cell carcinoma should be clear
May be more evident on cytologic preparations
Caution! It may be very difficult to differentiate these neoplasms, specially on frozen section
Pheochromocytoma has a nested and zellballen pattern with basophilic cytoplasm and bizarre, isolated, atypical nuclei
Pheochromocytoma has usually been diagnosed preoperatively
Caution!
Occasionally, these tumors have similar histopathological appearance
Adrenal cortical neoplasms may also have intranuclear inclusions
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree