KEY POINTS
Although advances have been made in percutaneous coronary intervention techniques for coronary artery disease, survival is superior with coronary artery bypass grafting in patients with left main disease, multivessel disease, and in diabetic patients.
Coronary artery bypass grafting has become increasingly safe, and improves late mortality in patients with left main or proximal left anterior descending disease, multivessel disease, and in patients with diabetes.
Despite the theoretical advantages, the superiority of off-pump coronary artery bypass to conventional coronary artery bypass grafting has not been clearly established and other factors likely dominate the overall outcome for either technique.
Although mechanical valves offer enhanced durability over tissue valve prosthesis, they require permanent systemic anticoagulation therapy to mitigate the risk of valve thrombosis and thromboembolic sequelae, and thus are associated with an increased risk of hemorrhagic complications.
Mitral valve repair is recommended over mitral valve replacement in the majority of patients with severe chronic mitral regurgitation. The decision to proceed with mitral valve repair is based on the skill and experience of the surgeon in performing repair, and on the location and type of mitral valve disease encountered at the time of operation.
Although open aortic valve replacement has traditionally been the only effective treatment in patients with severe calcific aortic stenosis, transcatheter aortic valve replacement is a developing technology that has proven beneficial for the treatment of aortic stenosis in seriously ill patients that had previously been deemed high risk or inoperable.
Mechanical circulatory support with newer generation continuous flow left ventricular assist devices has proven to be durable and effective both in bridging patients to transplant and as a means of “destination therapy” for patients who are not transplant candidates. Recent results for destination therapy have approached those of cardiac transplantation.
Performing a biatrial Cox-Maze lesion set results in freedom from atrial fibrillation in approximately 90% of patients and is superior to both catheter-ablation and more limited lesion sets for patients with persistent atrial fibrillation or enlarged left atria. Surgical ablation of atrial fibrillation is recommended for patients referred with concomitant valvular disease and those who have previously failed or are poor candidates for catheter-based approaches.
The preferred treatment for pericarditis depends on the underlying cause, although the disease typically follows a self-limited course and is best managed medically. Surgical pericardiectomy may have a role in treating relapsing pericarditis and, more commonly, chronic constrictive pericarditis.
Myxomas are the most common cardiac tumors, and, while benign, they should be promptly excised after diagnosis due to the risk of embolization, obstructive complications, and arrhythmias.
CARDIAC ASSESSMENT
As with any other field in medicine, history, and physical examination form the foundation for the evaluation of a patient with acquired heart disease requiring surgical intervention. Obtaining a complete history will help identify comorbid conditions and assist in delineating the operative risks and prognosis after surgery. Physical examination not only reveals factors that may increase the complexity of surgery, such as previous surgery or peripheral or cerebral vascular disease. These may influence the operative approach but also help guide the choice and sequencing of diagnostic studies. A complete assessment of the patient allows the surgeon to make educated decisions regarding the optimal treatment strategy for the patient.
Symptoms suggestive of heart disease include: chest discomfort, fatigue, edema, dyspnea, palpitations, and syncope. Adequate definition of these symptoms calls for a detailed history-taking paying particular attention to onset, intensity, radiation, duration, and exacerbating/alleviating factors. The demands on the heart are determined by its loading conditions and metabolic state of the body, and symptoms are commonly accentuated with physical exertion or postural changes.
Angina pectoris is the hallmark of coronary artery disease (CAD), but may occur with other cardiac pathologies which results in ischemia from a mismatch between the supply of oxygen by the coronary circulation and the metabolic demand of the myocardium. Typically, angina is described as tightness, heaviness, or dull pain, most frequently substernal in location, lasting for a few minutes. This discomfort may radiate to the left arm, neck, mandible, or epigastrium. It is most often provoked by activities that increase metabolic demand on the heart such as exercise, eating, and states of intense emotion, and is typically alleviated by rest or use of nitroglycerin. It is important to note that a significant number of patients with myocardial ischemia, particularly diabetics, females, and the elderly, may have “silent” angina or angina equivalents (dyspnea, diaphoresis, nausea, or fatigue). The overlap of these features with those of noncardiac etiologies such as costochondritis, biliary colic, gastroesophageal reflux disease, diffuse esophageal spasm, and peptic ulcer disease, to name a few, can lead to misdiagnosis.
Heart failure can occur in the left and/or right heart and respective symptoms arise from congestion of blood flow owing to the inadequacies of the cardiac pump function. Left heart failure manifests as dyspnea, usually with exertion. Orthopnea suggests worsened pulmonary congestion with increased venous return and these patients are not able to lie flat. Ascites, peripheral edema, and hepatomegaly reflect congestion in the systemic venous circulation and are prominent features of right heart failure. Peripheral edema can occur in right heart failure secondary to systemic venous congestion, or in left heart failure due to salt and fluid retention due to impaired renal perfusion. Patients with chronic suboptimal perfusion and oxygenation can also have digital clubbing and cyanosis.
It is difficult to implicate cardiac disease based solely on the presence of fatigue as it is a very nonspecific symptom. However, most cardiac pathologies do result in fatigue or exercise intolerance of some degree. It is important to differentiate fatigue from exertional dyspnea which some patients may describe as “fatigue.”
Dyspnea is another common symptom seen in many heart diseases. Although generally a late symptom in patients with valvular heart disease or cardiomyopathy, it may be a relatively early complaint in some patients, particularly those with mitral stenosis. As stated previously, dyspnea is also an anginal equivalent and may signal a myocardial ischemic episode. Many primary pulmonary disorders feature dyspnea as their cardinal symptom and should be evaluated simultaneously as the physiology of the heart and lungs are intimately related, and can have dramatic influences on each other.
Patients typically describe palpitations as a “skipped beat” or “racing heart”. Depending on the clinical context, such as occasional premature atrial or ventricular beats in otherwise healthy individuals, these may be benign. Clinically significant arrhythmias, however, require thorough investigation. Atrial fibrillation is the most common arrhythmia and can occur alone or with concomitant cardiac pathologies. It results in an irregular, and at times, rapid heartbeat. Concurrent symptoms such as angina or lightheadedness/syncope are particularly worrisome for life threatening arrhythmias such as ventricular tachycardia or ventricular fibrillation, particularly in those with preexisting heart failure or ischemic heart disease.
Syncope associated with heart disease results from abrupt reduction of cerebral perfusion. Many of the potential etiologies are serious, including sinus node dysfunction, atrioventricular-conduction abnormalities, malignant arrhythmias, aortic stenosis, and hypertrophic obstructive cardiomyopathy. Any episode of syncope warrants a thorough evaluation and search for the root cause. 1
In addition to a thorough inquiry regarding the above symptoms, it is important to obtain details about the patients’ medical/surgical history, family history, social habits (with regards to alcohol and tobacco use), current medications, and a focused review of systems, including an assessment of the patients’ functional status and frailty. Specific attention should be directed to the patients’ comorbidities which not only sheds light on the patients’ general health, but also helps delineate the risks if the patient were to undergo surgery. A strong family history of coronary artery disease, myocardial infarction, hypertension, or diabetes is of particular importance as they increase the individuals’ risks.
With regard to heart failure, functional capacity is strongly correlated with mortality. The New York Heart Association (NYHA) functional classification is the most widely used classification system in categorizing patients based on their functional status (Table 21-1). The NYHA classification has become a basis by which to assess patient characteristics in many studies to compare patient populations. Although less commonly used, the Canadian Cardiovascular Society (CCS) angina classification is also used to incorporate anginal symptoms into the functional assessment for prognostic value (Table 21-2).
| CLASS | DESCRIPTION |
|---|---|
| I | Physical activity not limited by symptoms: fatigue, palpitations, or dyspnea. |
| II | Comfortable at rest. Slight limitation of physical activity. Fatigue, palpitations, or dyspnea with ordinary physical activity. |
| III | Comfortable at rest. Marked limitation of physical activity. Fatigue, palpitations, or dyspnea with less than ordinary physical activity. |
| IV | Inability to carry out any physical activity. Symptoms may be present at rest and increase with activity. |
| CLASS | DESCRIPTION |
|---|---|
| I | Ordinary physical activity (walking, climbing stairs) does not cause angina. Angina occurs with strenuous, rapid, or prolonged exertion during work or recreation. |
| II | Slight limitation of ordinary activity. Angina occurs with climbing stairs rapidly, walking uphill in the wind, under emotional stress, in the cold, or after meals. Walking more than 2 blocks or climbing one flight of stairs causes angina. |
| III | Marked limitation of ordinary physical activity (climbing a flight of stairs or walking 1 to 2 blocks at a normal pace). |
| IV | Inability to carry out any physical activity without discomfort. Angina may be present at rest. |
The physical examination is an invaluable tool in directing further diagnostic studies and management of a patient with suspected heart disease. The astute clinician will detect subtle signs that may further characterize the underlying pathologic process.
The general appearance of a patient is important in the clinical assessment. A pale, diaphoretic, and obviously uncomfortable patient is more likely to be in a clinically critical condition than one who is conversing comfortably with an unremarkable demeanor. In addition to basic vital signs, particular attention should be directed to the patients’ mental status and skin (color, temperature, diaphoresis) as these may be reflective of the general adequacy of perfusion. Overall frailty and dementia have also been shown to be predictors of operative and late mortality.2
Palpation of the precordium may demonstrate deviations in the point of maximal impulse indicative of ventricular hypertrophy or parasternal heaves seen in right ventricular overload. Auscultation should be performed in a quiet environment as critical murmurs, rubs, or gallops may be subtle. Murmurs are characterized by their location, timing, quality, and radiation. They are typically secondary to valvular or other structural pathology and new findings require further investigation. A rub due to pericardial friction is specific and virtually pathognomonic for pericarditis. A third heart sound (S3) is generated by the rapid filling of a stiff ventricle and can be normal in young patients, but when present in older adults, is indicative of diastolic dysfunction and is pathologic. Increased contribution of the atrial pump function to ventricular filling may manifest as a fourth heart sound (S4) and is also suggestive of ventricular dysfunction.
Palpation of peripheral pulses is important not only to assess the adequacy of perfusion, but the burden of coronary artery disease often correlates with the degree of peripheral arterial disease. Discovery of carotid stenosis by auscultation for carotid bruits has significant implications for operative planning.
Heart disease will frequently have extracardiac manifestations and examination of the other organ systems should not be neglected. Auscultation of the lung fields may reveal rales in patients with pulmonary edema. The work of breathing may also be assessed simply by observing the patient. Jugular venous distention and hepatosplenomegaly are seen in right heart failure.
Approximately one half of the mortality in patients undergoing noncardiac surgery is due to complications which are cardiovascular in origin.3 The American College of Cardiology and American Heart Association have formed a joint task force to publish a consensus statement on guidelines and recommendations which was revised in 2007.4 The aim of these guidelines is to incorporate surgery-specific risks and patient characteristics to stratify patients in order to guide perioperative decision-making.
Surgical procedures have been categorized based on cardiovascular risk into low and moderate risk, and vascular procedures. Vascular procedures (aortic, peripheral vascular, and other major vascular surgery), likely due to both the nature of the procedures themselves as well as the associated cardiovascular pathology in many of these patients, carries the highest reported cardiac risk at more than 5%. Low risk procedures, including endoscopic procedures, superficial operations, cataract surgery, breast surgery, and ambulatory surgeries have a risk generally less than 1%. Intermediate risk procedures are: intraperitoneal and intrathoracic surgery, head and neck surgery, orthopedic procedures, and prostate surgery.
Patient characteristics can be classified by the status of the patients’ cardiac disease, comorbid conditions, and functional capacity. Patients are considered to be at major perioperative clinical risk if they have one or more of the following active cardiac conditions: unstable coronary syndrome, decompensated heart failure, significant arrhythmias, or severe valvular heart disease. In these patients, intensive evaluation and treatment prior to surgery (unless emergent) is warranted, even if the noncardiac surgery needs to be delayed or cancelled.
If the patient does not have any of the previously mentionedactive conditions, and is scheduled for a low risk surgery or if they have functional capacity greater than or equal to 4 metabolic equivalents (or METs), the official recommendation is to proceed with the planned operation. The previous guidelines contained intermediate and low cardiovascular risk profiles, but this has been replaced by cardiovascular risk factors in the update. These risk factors are: history of ischemic heart disease, history of prior or compensated heart failure, history of cerebrovascular disease, diabetes mellitus, and renal insufficiency. Based on the number of present risk factors and the surgery-specific risk, the guideline recommends pathways for further evaluation and risk management (Table 21-3).
| NUMBER OF RISK FACTORS* | RECOMMENDATION |
|---|---|
| 0 | Proceed with planned surgery. |
| 1–2 | Control HR and proceed with planned surgery or pursue further testing if it will change management. |
| 3–5 | Pursue further testing if it will advance management. |
| ANATOMY | CLASS OF RECOMMENDATION | LEVEL OF EVIDENCE |
|---|---|---|
| • LM | I | B |
| • 3-vessel +/− proximal LAD | I | B |
| • 2-vessel + proximal LAD | I | B |
| • 2-vessel – proximal LAD | IIa – with extensive ischemiaIIb – without extensive ischemia | B C |
| • Multivessel disease with DM | IIa (CABG preferred over PCI) | B |
| • Proximal LAD only | IIa – with LITA for long-term benefit | B |
| • 1-vessel – proximal LAD | III – Harm | B |
| • LV dysfunction | IIa – LVEF 35%–50%IIb – LVEF <35% without LM disease | B B |
| • Survivor of ischemia-mediated VT | I | B |
Electrocardiograms (ECGs) and chest X–rays are simple, noninvasive, and inexpensive diagnostic studies that are invaluable in the preoperative assessment of patients with cardiac pathology. ECGs can be useful in detecting old myocardial infarction, dilation or hypertrophy of cardiac chambers, arrhythmias, and conduction abnormalities. A stress ECG requires a patient to exercise to a target heart rate, and is used to help diagnose ischemic pathologies which may not be evident at rest.
A plain film of the chest can detect pulmonary pathology, sequelae of heart failure (e.g., pulmonary edema, cardiac enlargement, pleural effusions) as well as hardware from previous procedures such as, prosthetic valves, sternal wires, pacemakers, and defibrillators.
Echocardiography utilizes reflected sound waves to image the heart, and is used widely due to its noninvasive nature and low cost. It is the primary diagnostic tool used to evaluate structural diseases of the heart, including: valvular pathology, septal defects, cardiomyopathies, and cardiac masses. Echocardiography is indispensable in assessing surgical prosthetics such as valves, leads, or mechanical circulatory support devices. These examinations can be performed with M-mode imaging (motion along a single line) as well as 2-D and 3-D imaging depending on the graphical information required.
Doppler technology has become a standard addition to assess changes in flow patterns across both stenotic and regurgitant valves. Velocity measurements can be obtained to estimate pressure gradients across structures using the continuity equation. A common example would be the estimation of pulmonary arterial systolic pressure calculated from the regurgitant tricuspid jet profile during right ventricular systole.
Transthoracic echocardiography (TTE) requires no sedation and is generally performed with the patient in a slight left lateral decubitus position. Standardized views are obtained with the ultrasound probe placed in the apical, parasternal, subcostal, and suprasternal positions. The apical four-chamber view is a useful window for visualizing all four cardiac chambers simultaneously as well as the tricuspid and mitral valves. Other windows can be obtained to assess specific structures such as the individual valve anatomy or myocardial wall segments. Dobutamine-stress echocardiography is a study similar in idea to the stress ECG which utilizes a pharmacologic agent to assess the patient for ischemia or stress-induced valvular abnormalities.
A slightly invasive variant of this technology is transesophageal echocardiography (TEE) which takes advantage of the anatomic proximity of the heart to the esophagus. The exam is performed using a special endoscope with an ultrasound probe mounted on its end which is introduced orally into the esophagus under sedation. Posterior structures such as the mitral valve and left atrium are particularly well visualized. TEEs are frequently used intraoperatively during cardiothoracic surgery to assess global cardiac function, integrity of valve repairs and replacements, intracavitary thrombus and/or air, and aortic atherosclerosis or dissections which can have significant influences on operative strategy.
There are some new additions to the echocardiographic armamentarium which capitalize on the strengths of ultrasound imaging. Three dimensional TEE is playing an increasing role in the preoperative and intraoperative evaluation of patients with valvular heart disease and is particularly useful in the valuation of mitral regurgitation. Tissue Doppler imaging is based on principles akin to conventional Doppler echocardiography, but attention is directed to the myocardium itself as opposed to the motion of blood to quantify abnormalities in wall motion. Strain imaging with speckle-tracking echocardiography measures the actual deformation of the myocardium by following inhomogeneities inherent to the myocardium, and is a useful measure of myocardial function.
Although ECGs are useful, inexpensive, and safe, baseline abnormalities in the ECG may limit its diagnostic capacity. In particular, ventricular rhythms, bundle-branch blocks, left ventricular hypertrophy, drug effects, and baseline ST-segment depressions can make stress ECGs uninterpretable. In this setting, myocardial perfusion imaging (MPI) using radionuclides can be utilized to assess myocardial ischemia.
Thallium 201 (201Tl) was the initial radionuclide used for MPI, but due to its long half-life and relatively low photopeak, it has largely been replaced by Technetium-99m (sestamibi and tetrofosmin) which has more favorable characteristics. In the past, planar imaging with three separate two-dimensional views of the heart were obtained. Currently, it is more common to have the images acquired by single-photon emission computed tomography (SPECT) technology which detects emitted photons from 180- to 360-degrees around the patients. The signals are then processed to reconstruct multiple slices which together provide a three-dimensional view. The amount of uptake at both rest and stressed states are compared to assess ischemia and viability of the myocardium. The distribution of radionuclides depend on perfusion and therefore areas which show uptake at rest, but not during stress are concerning for ischemia.
Territories that do not show uptake at rest or during stress are likely to be nonviable scar. The sensitivity and specificity of exercise SPECT are 90% and 70%, respectively.5
The image acquisition may also be gated to a simultaneously obtained ECG to assess global ventricular function. The endocardial and epicardial borders (as delineated by radionuclide uptake) are detected throughout the cardiac cycle and the ejection fraction, along with end-systolic and end-diastolic volumes, can be calculated. This study is also useful in revealing hypokinetic segments of the myocardium.
One of the most significant weaknesses of SPECT imaging is that it shows regional ischemia well, but does not adequately detect global or “balanced” ischemia which can occur with diffuse CAD. Positron emission tomography (PET) scans have been used due to its ability to obtain absolute quantitative data on both myocardial perfusion and metabolism. Tracers used in PET scans can be divided into those that assess perfusion (Oxygen-15, Nitrogen-13, and Rubidiuim-82) and those that assess metabolism (Carbon-11 and Fluorine-18). The specificity of PET in detecting CAD is better than SPECT at 86% due to its superior spatial resolution.6
Magnetic resonance imaging (MRI) has a wide variety of uses in cardiac imaging depending on the pulse sequence and signal weighting. Cine-loop of the heart throughout the cardiac cycles can yield information on global chamber function and valvular pathologies. The differential response of normal and ischemic myocardium to certain pulse sequences allows imaging of myocardial perfusion using MRI. Use of contrast agents such as gadolinium can enhance scar tissue and are very useful in viability assessment. Myocardial strain imaging can also be performed using newer technologies taking advantage of radio-frequency tagging of the myocardium which deforms with the tissue and can be followed throughout the cardiac cycle.
Cardiac catheterization involves access to the cardiac chambers and great vessels with a peripherally inserted catheter under fluoroscopic guidance. It is a versatile tool used for diagnostic purposes of: cardiac chamber pressures, valvular abnormalities, wall motion assessment, and coronary artery anatomy. While some of these roles are being replaced by less invasive techniques mentioned previously, cardiac catheterization studies continue to be widely performed and is the gold standard for the assessment of coronary artery disease.
A left-heart catheterization is performed by percutaneous access of the femoral, or less commonly, the radial artery. Under fluoroscopic guidance, the catheter is threaded into the aorta where a contrast aortogram may be performed. Coronary angiography requires manipulation of this catheter into the coronary ostia where contrast is directly injected. With advancement of the catheter retrograde past the aortic valve, left ventricular pressures can be obtained. This pressure is used to calculate pressure gradients across the aortic valve which becomes particularly important in the evaluation of aortic stenosis. Again, contrast injection will result in a ventriculogram used to estimate ejection fraction and visualize hypokinetic segments of the walls. Inappropriate retrograde leakage of contrast may indicate insufficiency of the aortic and/or mitral valves.
Right heart catheterization is performed through a peripheral vein and the catheter is threaded into the right atrium. Right-sided pressures and structures are assessed in a similar fashion as in the left heart. Extension of the catheter into the pulmonary artery allows measurement of the pulmonary artery pressures as well as the pulmonary capillary wedge pressure (reflecting left ventricular end diastolic pressure) with an occlusive balloon.
In addition to these measurements, cardiac output can be measured using thermodilution or by the Fick method using oxygen saturations of blood sampled from the various locations during the procedure.
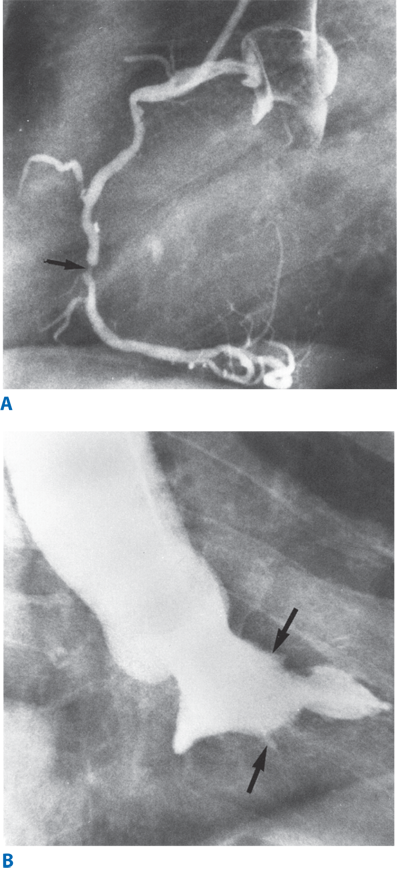
Coronary angiography provides information on hemodynamically significant stenoses in the coronary circulation as well as an anatomical roadmap for surgeons to planning revascularization. (Fig. 21-1A & B) A stenosis is considered to be significant if it narrows the lumen of the artery by 70% (or 50% in the case of left main coronary artery). There is some variability in the coronary arterial anatomy with the posterior descending artery being supplied by the right coronary artery in approximately 80% of patients (right dominant), or the left coronary artery in approximately 15% of patients (left dominant). The remaining patients have a codominant circulation where the posterior descending artery is supplied by both the right and left coronaries.
An advantage of catheterization is that it offers an opportunity for interventional therapy of coronary artery disease, arrhythmias, valvular abnormalities, and other structural defects of the heart. Cardiac catheterization is generally safe, but being an invasive procedure, it is associated with complications. Overall mortality is 0.11%, and total rate of major complications, including: MI, stroke, arrhythmia, vascular injury, contrast reaction, hemodynamic instability, and cardiac perforation is usually <2%.7
Multislice computed tomography (CT) imaging can be used to assess the coronary vasculature. The coronary calcium score is an index developed to quantify the degree of coronary atherosclerotic burden by measuring Hounsfield units in a noncontrast cardiac CT. Although this technique is quite sensitive for angiographic stenoses >50%, it remains fairly nonspecific as calcification often precedes significant luminal narrowing.8 CT coronary angiography using intravenous contrast is also utilized clinically to assess coronary pathology and is particularly useful in the emergency room to perform a “triple rule-out” for acute coronary events, pulmonary embolism, and aortic dissection in patients who present with undifferentiated chest pain. LV ejection fraction may be measured by this technique, and together with the degree of coronary stenoses, incremental prognostic value has been demonstrated in addition to routine clinical predictors.9
EXTRACORPOREAL PERFUSION
Prior to the development of extracorporeal perfusion, heart surgery was rarely performed and was limited to brief periods of asystole and hypothermia. The need for a bloodless operating field, while maintaining perfusion of heart and other organs, was evident.
John Gibbon’s motivation to develop a means for extracorporeal perfusion came from a desire to safely open the pulmonary artery in a patient who suffered from a pulmonary embolus following cholecystectomy. After numerous experimental iterations, Gibbon’s cardiopulmonary bypass machine was first used clinically in 1953 to repair a large atrial septal defect in an 18-year-old female.10
Although Gibbon is credited for its invention, the development of modern cardiopulmonary bypass (CPB) is a culmination of the work of many investigators throughout the world. The early bubble oxygenators have evolved into the modern membrane oxygenators. The search for an ideal biocompatible material which minimizes the inflammatory cascade initiated by the contact of blood with the circuit components continues to this day.
The basic CPB circuit consists of the venous cannulae, a venous reservoir, pump, oxygenator, filter, and the arterial cannula.
Anticoagulation is required during CPB, and 300 to 400 units/kg of heparin is given to increase the activated clotting time (ACT) to greater than 450 seconds. Once adequate anticoagulation is achieved, arterial cannulation is performed through a purse-string suture, or through a side graft which is sewn on to the native artery. The distal ascending aorta is the most common site of cannulation, but there may be concern for atheroembolization when the aorta is atherosclerotic. Other sites of cannulation include the femoral artery, axillary artery, or the distal aortic arch. Venous cannulation is performed through purse-string sutures placed on the right atrium either for a single cannula or for two separate cannulae extending into the superior and inferior vena cava, respectively. Alternatively, the venous cannula may be inserted from the femoral vein and advanced into the right atrium.
Effective communication between the surgeon, the anesthesiologist, and the perfusionist is mandatory for effective cardiopulmonary bypass. Once the appropriate cannulations and connections are complete, CPB is commenced. Venous return is initiated followed by arterial flow while monitoring systemic blood pressures. At normothermia, the flow required is approximately 2.4 L/min/m2, but with hypothermia, oxygen consumption is reduced by 50% for every 10°C drop in temperature, and a flow of only 1L/min/m2 is required at 18°C. Once the heart is decompressed and hemodynamics are acceptable, ventilation is stopped. The oxygenator is adjusted to maintain a PaO2 of 150 mm Hg and normocarbia. Blood can also be filtered and returned through vents that are placed in the heart or through the cardiotomy suction used to aspirate blood from the surgical field.
When the cardiac procedure is complete, the patient is rewarmed, the lungs ventilated, and the heart defibrillated if needed. The venous return to the CPB machine is gradually reduced allowing the heart to fill. The pump is also slowed while hemodynamics and global cardiac function are assessed with a TEE probe. Inotropic and vasopressor support may be used to augment cardiac function and treat hypotension. Once CPB has been stopped and stable hemodynamics achieved, the cannulae are removed. The heparin anticoagulation is reversed with protamine and hemostasis is achieved.11
Cardiopulmonary bypass has a number of deleterious effects as various intertwining processes result in derangements in hemostasis, systemic inflammatory response, and end-organ function.
Anticoagulation prior to the commencement of CPB is required as contact of blood with the artificial surfaces of the circuit can initiate a thrombogenic cascade. Generation of thrombin plays a major role in both thrombotic and bleeding phenomena during CPB. The endothelium which normally regulates the fine balance between procoagulant and anticoagulant pathways is perturbed. Fibrinogen is consumed rapidly as thrombin converts fibrinogen to fibrin while fibrinolytic mechanisms (initiated by the activated endothelium) degrade the fibrin macromolecules. Platelets are activated by the converging hemostatic pathways and are consumed.
The response of the humoral and cellular immune systems partly overlap with the hemostatic pathways. The classic and alternative complement pathways are activated by CPB generating powerful chemotaxic molecules and anaphylatoxins. Monocytes, platelets, and neutrophils are activated releasing acute inflammatory mediators and cytokines which persist even after conclusion of CPB.12 These inflammatory cells also produce reactive oxidants which may have cytotoxic effects.
The large quantity of unfractionated heparin used during cardiac surgery predisposes patients to developing heparin induced thrombocytopenia (HIT) with an incidence of 1% to 2%. Platelet factor-4 (PF4) is produced by platelets and avidly binds to heparin to form a heparin-PF4 complex which can be antigenic in some patients binding IgG. The IgG-heparin-PF4 complex can bind to platelets which causes release of more PF4, perpetuating the process. The earliest sign is a sudden drop of more than 50% of the platelet count, and HIT can be confirmed with an ELISA or serotonin release assay. Of the patients with HIT, 20% to 50% of patients develop thromboses in arterial or venous beds, designated as heparin induced thrombocytopenia and thrombosis (HITT), which can be life-threatening.13
The etiology of end-organ dysfunction resulting from extracorporeal circulation can mostly be categorized into one of three mechanisms. Although cardiac output and blood pressure are monitored carefully during CPB, they are surrogates for regional perfusion and cannot detect end-organ hypoperfusion directly. This can be a problem particularly with the cerebral, renal, and mesenteric circulations. With manipulation of diseased vessels and dysregulation of the native coagulation system, macroscopic and microscopic emboli are a concern despite various strategies to minimize this problem. Activated cells and circulating cytotoxic products of the immune response may cause microvascular injury and edema of other organs manifesting as neurocognitive deficits, respiratory failure, and renal injury.14
During CPB, pharmacologic agents in cardioplegic solutions may be delivered into the coronary circulation to arrest the heart allowing for a still operating target and improved myocardial protection. The most common cardioplegia consists of potassium-rich solutions that can be mixed with autologous blood and are delivered into the coronary circulation. Antegrade cardioplegia is delivered into the root of a cross-clamped aorta or directly into the individual coronary ostial via specialized catheters. A retrograde cardioplegia catheter is a balloon-cuffed catheter that is placed through the right atrium into the coronary sinus and is used to perfuse the coronary circulation in the opposite direction through the venous circulation. This has the advantage of more uniform distribution in patients with diffuse coronary artery disease and is not dependent on a competent aortic valve for delivery.
There are controversies regarding the method (antegrade retrograde vs. both), type (crystalloid vs. blood), temperature (cold vs. warm vs. tepid), and interval (continuous vs. intermittent) of cardioplegia delivery. The optimal combination is beyond the scope of this text. However, most surgeons in the United States favor cold blood potassium cardioplegia.
CORONARY ARTERY DISEASE
The Vineberg operation, one of the initial attempts at surgical revascularization of the myocardium, was first performed in 1950s. This procedure involved implantation of the internal thoracic artery directly into the myocardium itself. While some patients were relieved of their anginal symptoms, this resulted in very little increase in coronary flow and was supplanted by methods to restore flow directly. Coronary endarterectomy was introduced by Longmire during this time period, but was met with high rates of restenosis and occlusion. The use of vein patches to repair the arteriotomy sites was described by Senning in 1961. The first saphenous vein coronary artery bypass grafting (CABG) was performed by Sabiston in 1962, but was popularized by Favalaro in 1967. In 1968, the internal thoracic artery was introduced as a bypass conduit by Green who used it to bypass the left anterior descending coronary artery.15
Atherosclerotic stenoses are the primary mechanism of coronary artery disease (CAD). The pathophysiologic process is initiated with vascular endothelial injury and is potentiated by inflammatory mechanisms, circulating lipids, toxins, and other vasoactive agents in the blood. Macrophages and platelets are attracted to this area of endothelial dysfunction inciting a local inflammatory response. During this process, macrophages infiltrate into the intimal layers and accumulate cholesterol-containing low-density lipoproteins. The growth factors secreted promote proliferation of smooth muscle cells within the intima and media of the arteries. Together with the accumulation of the lipid-laden macrophages, the smooth muscle hyperplasia results in an atheroma and subsequently stenosis of the vessel. These atheromas have a fibrous cap which may rupture, exposing the underlying cells and extracellular matrix which are very prothrombotic. Acute plaque rupture and thrombus formation is thought to be the main pathophysiologic mechanism responsible for acute coronary syndromes.16,17,18
Prior to the establishment of modern management strategies, the annual mortality rated from ischemic heart disease was quoted to be around 4% by the Framingham study. Since then, risk factor modification along with use of medications, such as aspirin and β-blockers, has dramatically improved survival.
The major risk factors of atherosclerosis include: age, cigarette smoking, hypertension, dyslipidemias, sedentary lifestyles, obesity, and diabetes. Likely due to increased public awareness and aggressive medical management, these risk factors (with the exception of glucose intolerance and obesity) have recently been on the decline.
Current guidelines outlined in the AHA/ACC consensus statement summarizes secondary prevention recommendations. Class I recommendations are: smoking cessation and avoidance of environmental tobacco exposure, blood pressure control to under 140/90 mm Hg (under 130/80 mm Hg in those with diabetes or chronic kidney disease), LDL cholesterol levels less than 100 mg/dL, aspirin therapy in all patients without contraindications, BMI target of less than 25 kg/m2, diabetes management with target HbA1c <7%, and encouragement of daily aerobic exercise routines. Beta-blockers are to be considered in patients with LV dysfunction and following MI or ACS. Renin-angiogensin-aldosterone system blockade in patients with hypertension, LV dysfunction, diabetes, or chronic kidney disease should also be considered.19
Patients with CAD may have a spectrum of presentations, including angina pectoris, myocardial infarction, ischemic heart failure, arrhythmias, and sudden death.
Angina pectoris is the pain or discomfort caused by myocardial ischemia and is typically substernal and may radiate to the left upper extremity, left neck, or epigastrium. The variety of presentations can make myocardial ischemia difficult to diagnose. Characteristics of chest pain that make myocardial ischemia less likely include: pleuritic chest pain, pain reproducible by movement or palpation, or brief episodes lasting only seconds. Typical angina is relieved by rest and/or use of sublingual nitroglycerin. Differential diagnoses to be considered include, but are not limited to: musculoskeletal pain, pulmonary disorders, esophageal spasm, pericarditis, aortic dissection, gastroesophageal reflux, neuropathic pain, and anxiety.
Myocardial infarction is a serious consequence of CAD occurring when ischemia results in myocardial necrosis. This may be silent and need not be preceded by angina. Necrosis may result in disruption of the myocardial integrity leading to devastating conditions such as intracardiac shunts from ventricular septal defects, acute valvular regurgitation from rupture of necrotic papillary muscles, and cardiac aneurysms which have the catastrophic potential to rupture.
The ischemic insults from CAD may lead to congestive heart failure. The initial myocardial damage sets off a cascade of responses, both local and systemic. Over time, these changes can cause deleterious myocardial loading and abnormal neurohumoral responses that result in pathologic remodeling of the heart. Heart failure should be suspected in patients who present dyspnea, orthopnea, fatigue, and edema.
Arrhythmias may also be a sequela of CAD. Ischemic etiologies should be investigated in patients who present with new arrhythmias. CAD may result in arrhythmias following an acute MI or as the result of ultrastructural and electrophysiologic remodeling secondary to chronic ischemic heart disease. Ischemia of the electrical conduction system may be seen as the new onset complete or partial atrioventricular conduction blocks.
A focused history and physical examination is essential with particular attention directed to the signs, symptoms, and clinical manifestations mentioned previously. The patient’s functional status is of importance not only because it is a component of preoperative risk assessment, but also because quality of life improvement and symptomatic relief are both goals of surgical therapy.
Coronary angiography is the primary diagnostic tool. The coronary anatomy and degrees of stenoses are delineated allowing for planning of surgical revascularization.
Noninvasive diagnostic studies, in combination with provocative maneuvers (exercise or pharmacologic agents) offer information regarding the functional significance of ischemic disease. A stress ECG is frequently used as a screening tool with a high sensitivity. The positive predict value is 90% in patients with ST-segment depression >1mm. This test however, requires patients to achieve a certain elevation in their heart rate, and is therefore not suitable for those that cannot achieve this goal. Furthermore, baseline ECG abnormalities may render it impossible to detect typical ischemic changes with stress.
Echocardiography and nuclear imaging may be performed under pharmacologic stress (with dobutamine or dipyridamole) to assess reversible ischemia and myocardial viability. Technetium-99m or thallium-201 perfusion scans have an average sensitivity and specificity of 90% and 75%, respectively. Stress echocardiography has a similar sensitivity and specificity of approximately 85%.20 These studies also have the ability to assess global ventricular function in terms of LV ejection fraction which can be used to determine operative risk.
CORONARY ARTERY BYPASS GRAFTING
A joint committee established by the American College of Cardiology and the American Heart Association have published guidelines for surgical revascularization (CABG) in CAD. The indications, categorized by presentation and angiographic disease burden as well as by treatment intention (survival improvement and symptom relief), are summarized later (Tables 21-4,5,6).21
| ANATOMY ASSOCIATED SYMPTOMS | CLASS OF RECOMMENDATION | LEVEL OF EVIDENCE |
|---|---|---|
| • Unacceptable angina with presence of ≥1 stenoses amenable to revascularization despite medical treatment | I | A |
| • Complex 3-vessel CAD +/- proximal LAD involvement | IIa (CABG preferred over PCI) | B |
| • Unacceptable angina with presence of ≥1 stenoses amenable to revascularization but medical treatment is not possible | IIa | C |
| • Previous CABG with ≥1 stenoses associated with ischemia and angina despite medical treatment | IIb | C |
| CLINICAL SETTING | CLASS OF RECOMMENDATION | LEVEL OF EVIDENCE |
|---|---|---|
| Emergent CABG following acute MI | ||
• Failure or inability to perform PCI, anatomy suitable for CABG, persistent ischemia/hemodynamic instability refractory to nonsurgical therapy • Patients undergoing surgical repair of postinfarction complication (e.g.,VSD, papillary or free wall rupture) • Cardiogenic shock with anatomy suitable for CABG • Life-threatening ventricular arrhythmia, ischemic in origin, with 3-vessel disease or >50% LM stenosis • Multivessel disease, recurrent angina or MI within 48 hrs of STEMI (instead of a more delayed strategy) • Age >75 with ST-elevation or new left bundle branch block who are suitable for revascularization, regardless of time between MI and cardiogenic shock. • Persistent angina with only small area of viable myocardium | I I I I IIa IIa III – Harm | B B B C B B C |
| Survival from sudden cardiac death or sustained VT | ||
• If thought to be due to significant CAD (amenable to revascularization). • If only scar present, and no evidence of ischemia | I III- Harm | B C |
| Patients undergoing concomitant non-coronary cardiac surgery | ||
• In presence of significant CAD (>50% LM stenosis or >70% stenosis of another major coronary artery. • LITA graft to significantly narrowed LAD or CABG of moderately diseased (>50% stenosis) coronary artery. | I IIa | C C |
| Emergent CABG after failed PCI | ||
• Ongoing ischemia, threatened occlusion with substantial myocardium at risk, or hemodynamic compromise (without coagulopathy or previous sternotomy). • Retrieval of foreign bodies (from PCI) or hemodynamic compromise with coagulopathy and without previous sternotomy. • Absence of ischemia or threatened occlusion | I IIa III – Harm | B C C |
| MURMUR | CONDITION | MECHANISM/ETIOLOGY |
|---|---|---|
| Systolic murmurs | ||
| Holosystolic (pansystolic) | VSD | Flow between chambers that have widely different pressures throughout systole |
| Mid-systolic (systolic ejection) | High flow rate, MS, MR, TS, TI | Often crescendo-decrescendo in configuration; occur as blood is ejected into the left and right ventricular outflow tracts |
| Early systolic | Early TI, acute MR | Less common |
| Mid to late systolic | MR, MVP | Soft to moderate high-pitched murmurs at the LV apex; often due to apical tethering and malcoaptation of MV leaflets; an associated click indicates prolapse of the MV leaflets |
| Diastolic murmurs | ||
| Early high-pitched | AI, PR | Generally decrescendo in configuration; occur when the associated ventricular pressure drops sufficiently below that of the outflow tract |
| Mid-diastolic | MS, TS, PDA*, VSD*, ASD* | Due to a relative disproportion between valve orifice size and diastolic blood flow volume; seen in normal MV and TV with increased diastolic blood flow associated with these conditions* |
| Presystolic | MS, TS | Occur during the period of ventricular filling that follows atrial contraction (i.e.,only occur in sinus rhythm) |
| Continuous murmurs | ||
| Systolic and diastolic | PDA | Uncommon, due to shunts that persist through the end of systole and the some or all of diastole |
In recent years, there have been multiple prospective randomized, controlled trials as well as retrospective studies looking at the comparative effectiveness of percutaneous coronary interventions (PCI) and CABG. Some of the representative studies are summarized here.
A retrospective review of 59,314 patients in two of New York’s registry with multivessel (2 or more) coronary disease was performed. Of these, 37,212 patients received a CABG and the others underwent a PCI. After adjusting by means of proportional-hazards methods, CABG was associated with higher adjusted rates of long-term survival than PCI.22
An international multicenter randomized controlled trial of 988 patients (n = 488 PCI, n = 500 CABG) with multivessel CAD was performed to compare revascularization strategies. At 2-year median follow-up, the PCI group had significantly higher rates of repeat revascularizations and mortality compared to the CABG group (incidence of nonfatal Q-wave myocardial infarctions were similar in both groups). The median follow-up was extended to 6-years, and a survival advantage persisted in the CABG group over the PCI group.23
Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX Trial, 2009)
Revascularization strategies, CABG vs. PCI, were compared in a 1:1 randomized prospective trial of 1800 patients with high risk coronary artery disease (left-main or triple-vessel disease). Rates of requirement for repeat revascularization and major adverse cardiac or cerebrovascular events at 12-months were lower in the CABG patients (5.9% and 12.4%, respectively) compared to PCI patients (13.5% and 17.8%, respectively). No difference in mortality was seen between the groups at 12-months.24
The ACCF and STS Database Collaboration on the Comparative Effectiveness of Revascularization Strategies (ASCERT Study, 2012)
This study, performed by collaboration of the American College of Cardiology Foundation and the Society of Thoracic Surgeons, reviewed their respective national databases of patients over the age of 65 who had multivessel coronary disease (excluding those with left main disease). CABG was performed on 86,244 patients and 103,549 underwent PCI. There was no difference in adjusted mortality at 1 year, but there was a significantly lower mortality with CABG than PCI at 4 years.25
PCI technology has improved over time and rates of periprocedural adverse events have decreased significantly. Management strategies must be tailored to the individual patient’s clinical status and context, but CABG maintains improved long-term outcome and remains the standard of care for left-main and multivessel coronary artery disease.
The most important criterion in conduit selection is graft patency. The conduit with the highest patency rate (98% at 5 years and 85%–90% at 10 years) is the internal thoracic artery which is most commonly left attached proximally to the subclavian artery (although occasionally used as a free graft) and anastomosed distally to the target coronary artery.26,27 The use of both internal thoracic arteries has been shown to increase event-free survival in a number of studies.28,29
The greater saphenous vein can be harvested using an open or endoscopic technique. In the open technique, the initial incision is made along the course of the vein on the medial aspect of the lower extremity. The vein is harvested with meticulous attention directed towards minimizing manipulation of the vein itself. The incision may be continuous or bridged in an attempt to decrease the size of the incision, but multiple bridged incisions may have the potential risk of increased conduit manipulation during harvest. Endoscopic harvest is performed by making a small incision just above and medial to the knee where the endoscope is inserted. Side branches are cauterized under endoscopic visualization using bipolar electrocautery until dissection is carried proximally until the required length of vein is mobilized. A proximal counterincision is then made to extract the venous conduit which is prepared in the standard fashion.
The radial artery is another frequently used conduit. After confirmation of ulnar collateral flow to the hand by the clinical Allen’s test or a duplex ultrasound study, an incision is made from a point just proximal to the radial styloid process ending just medial and distal to the biceps tendon on the nondominant hand. With lateral retraction of the brachioradialis muscle, the radial artery is dissected sharply with care to avoid injury to the cutaneous nerves in this area and minimize manipulation of the artery itself. This artery can also be harvested using an endoscopic technique.
Many studies have looked at the patency rates of the radial artery graft in comparison to the saphenous vein graft. Although some studies have resulted in equivocal data, general consensus favors the use of radial arterial grafts over vein grafts with 5 year patency rates of 98% and 86%, respectively.30,31
From a historical perspective, the anterior circulation (left main or left anterior descending artery) is generally bypassed using the internal thoracic artery and the lateral (circumflex artery) or inferior (right coronary artery) territories are bypassed using a saphenous vein or radial artery graft. These conduits may be combined to form a composite T- or Y-graft, or sewn to multiple targets as sequential grafts. Since patency is best with arterial grafts, recent data havesuggested that the best long term results are achieved with multiple or all-arterial revascularization, particularly in patients >70 years of age.32,33 Other conduits such as the gastroepiploic arteries, lesser saphenous veins, and cephalic veins have been described, but are not widely used and will not be discussed here.
Traditionally, CABGs are performed with the patient lying supine through a median sternotomy. After the patient is heparinized, cardiopulmonary bypass is initiated. The aorta is cross-clamped and cardioplegia is delivered. Once adequate myocardial protection has been achieved, coronary arteriotomies are made and distal anastomoses are performed using Prolene suture. (Fig. 21-2A & B) The proximal anastomoses are then performed directly onto the ascending aorta or onto preexisting grafts. It is important to note that significant coronary stenoses can cause differential distribution of cardioplegia and myocardial protection. It is therefore recommended to use retrograde cardioplegia or to revascularize the area with the most concern for ischemia first, and give cardioplegia down the completed graft. The left internal thoracic artery to left anterior descending (LAD) graft is frequently performed last to avoid kinking or disruption of this important bypass. Once all grafts are in place, the patient is weaned from bypass. During this time, the heart is monitored closely by direct visual inspection, and transesophageal echocardiography to detect abnormalities which may signify inadequate revascularization or technical problems with the bypasses. Upon confirmation of hemostasis, chest tubes are placed, the sternum is approximated with sternal wires, and the incisions are closed.
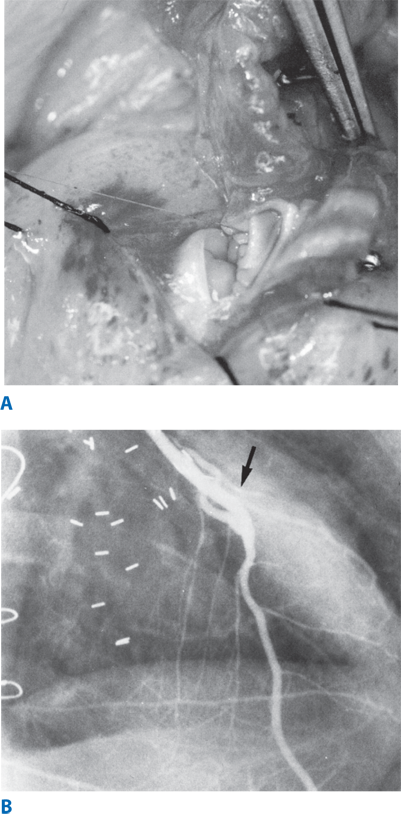
Figure 21-2.
Coronary artery bypass grafting. A. Intraoperative photograph of the distal anastomoses performed between the left internal thoracic artery and left anterior descending coronary artery with a continuous 8-0 suture. B. Fifteen-year follow-up coronary angiogram of a left internal thoracic artery to left anterior descending coronary artery bypass demonstrating a widely patent free of any significant atherosclerotic stenosis. Anastomotic site is shown by the arrow.
Several early randomized trials have shown improved survival in patients who receive a CABG as opposed to medical therapy.34,35,36 A propensity-matched study identified that CABG greatly benefited patients with LV dysfunction and left main stenosis >50% compared to medical management.37 The Bypass Angioplasty Revascularization Investigation (BARI) trial demonstrated impressively superior results with CABG compared to PCI in terms of 5-year cardiac mortality (5.8% vs. 20.6%) in patients with diabetes in addition to CAD.38 In a study examining the benefits of CABG over medical management for specific CAD distributions, survival was better in patients with proximal LAD stenoses, regardless of the number of diseased vessels.39 In general, these studies show survival rates of over 90% at 5 years and approximately 75% at 10 years following CABG.
The mortality and morbidity of the procedure itself has changed over time. Data from the Society of Thoracic Surgeons (STS) database accounts for 1,497,254 patients who underwent a solitary CABG from 2000 to 2009. The mortality rate of CABGs have improved significantly from 2.4% in 2000 to 1.9% in 2009 despite the relatively constant predicted mortality rate of around 2.3%. In parallel with this, postoperative complication rates have decreased as: stroke (1.6%–1.2%), bleeding requiring reoperation (2.4%–2.2%), and deep sternal wound infection (0.59%–0.37%).40
There are marked improvements in the functional status of patients receiving CABG. Patients’ 6-minute walk test distances were significantly increased 2 years postoperatively compared to their preoperative assessment.41 After 10 years, 54% of patients were free of chest pain and 31% were free of dyspnea.42
To avoid the adverse consequences of cardiopulmonary bypass, off-pump coronary artery bypass (OPCAB) was developed and has been adopted in some centers over the past two decades.
With OPCAB the heart is left beating. Performing anastomoses on the beating heart requires the use of myocardial stabilization devices which help portions of the epicardial surface to remain relatively immobile while the anastomoses are being performed. (Fig. 21-3) Carbon dioxide blower-misters are also used to clear blood from the operative site and improve visualization.
Apical suction devices are used to aid in exposure, particularly of the lateral and inferior vessels. Many creative maneuvers have been developed, including patient repositioning, opening the right pleural space to allow for cardiac displacement, and creation of a pericardial cradle to minimize compromise of cardiac function while exposing the various surfaces of the heart. Temporary proximal occlusion of the coronary artery being grafted is necessary to provide a bloodless target. This occlusion causes temporary ischemia, and if not tolerated, coronary shunts can be employed.
The superiority of OPCAB over on-pump CABG remains a controversy despite the large body of literature on this topic. A pooled analysis of two randomized trials, the Beating Heart Against Cardioplegic Arrest Studies (BHACAS 1 & 2), is one of several studies that have touted lower short term mortality rates with the off-pump compared to the on-pump technique.43,44,45 Other studies, however, have demonstrated equivocal or contrary results.46,47 Furthermore, the recent prospective and much larger ROOBY (Randomized On/Off Bypass) trial showed increased rates of adverse cardiac events with OPCAB compared to conventional CABG.48 Despite the initial enthusiasm for the theoretical advantages of avoiding cardiopulmonary bypass, consistent benefits in clinical outcome have not been observed. There does seem to be a more or less uniform trend towards decreased perioperative blood product transfusions with OPCAB compared to on-pump CABG. In terms of other measures of early outcome, postoperative renal failure, stroke, and acute MI, the superiority of OPCAB has been unclear.47,49,50
There have been questions whether the technical challenge of sewing on a beating heart leads to increased rates of graft occlusion following an OPCAB. The higher cardiac morbidity in the ROOBY trial was associated with decreased 1-year angiographic patency rates.48 However, studies with contrasting findings exist, quoting equivalent rates of graft patency for OPCAB usage.51,52 The broad variety in results may be suggestive that other factors (e.g.,surgeon skill, technical difficulty, patient factors) may be dominating the outcome rather than the use or avoidance of cardiopulmonary bypass.53 After almost two decades, OPCAB has not been widely adopted and remains less than 2% of all CABG procedures in the United States.
As an extension of the off-pump coronary revascularization technique, minimally invasive direct coronary artery bypass (MIDCAB) has been described. MIDCAB is performed using a left anterior mini-thoracotomy through which mobilization of the left internal thoracic and direct in situ anastomosis to the leftanterior descending artery (or its diagonal branches) is performed. This technique is primarily applicable to single-vessel disease, although reports of multivessel revascularizations do exist.
A review of 411 patients undergoing MIDCAB quotes an operative mortality >1%. In this study, all patients received revascularization of the LAD only, regardless of the number of diseased vessels. The 3-year mortality in patients with single-vessel disease following a MIDCAB was 3.1%, which was, not surprisingly, lower than those with multivessel disease (8.7%).54
There is an inherent selection bias in retrospective reviews comparing MIDCAB to OPCAB or conventional CABG as MIDCAB patients tend to have less extensive disease. Because of this, there have been multiple randomized controlled trials looking at the efficacy of MIDCAB compared to PCI. A meta-analysis of 5 randomized prospective trials comparing PCI to MIDCAB revascularization of isolated proximal left anterior descending artery demonstrated comparable results in terms of mortality, MI, and repeat revascularization requirement. It is worth noting however, that only one of these trials used drug-eluting stents (DES) in the PCI arm.55 Hong et al showed similar efficacy with MIDCAB and DES PCI, and when this study was excluded from the meta-analysis, superiority of MIDCAB to PCI in regards to mortality, MI, and repeat revascularizations was seen.56 Although no further prospective trials have been performed to compare DES PCI and MIDCAB in this patient cohort, a retrospective review of 186 patients has demonstrated significantly higher rates of angina recurrence and major adverse cardiac events in the DES PCI group.57
With the advent of robotic surgical technology allowing stereoscopic visualization and increased instrument dexterity, total endoscopic coronary artery bypass (TECAB) has become possible. In July of 2004, the da Vinci robotic surgical system received FDA approval for use in coronary anastomoses. Extracorporeal circulation with peripheral cannulation has been used in earlier reports, but the development of mechanical stabilizers has provided the ability to perform the internal thoracic artery harvest and coronary anastomosis off-pump with use of the robotic arms only. Several studies have looked at the feasibility of TECAB, and have shown acceptable results, but this procedure has not been adopted by most surgeons due to its steep learning curve, longer operative times, and lack of demonstrable clinical benefit.58,59,60
With the continually increasing collaboration between cardiothoracic surgeons and interventional cardiologists, hybrid coronary revascularization (HCR) combining a minimally invasive surgical technique (MIDCAB or TECAB) with PCI has become a reality. This capitalizes on a major advantage of both treatments, utilizing the durable left internal thoracic artery to left anterior descending coronary artery bypass while treating other stenoses with PCI obviating the need for a large surgical incision or cardiopulmonary bypass. HCR is not without its downsides as there are some concerns with this approach since aggressive anti-platelet therapy is required with PCI and may increase the hemorrhagic complications of surgical revascularization. A small study comparing HCR to OPCAB showed comparable graft patency and decreased hospital stay with HCR without an increase in complication rates.61 There are, however, some studies that have reported increased rates of requirement for re-intervention in patients undergoing HCR, and this aspect requires further study.62,63 These procedures have not gained widespread acceptance and their clinical value remains a matter of debate.
Despite the advancement of technology and revascularization strategies, patients with end-stage coronary artery disease may not be amenable to complete revascularization. Transmyocardial laser revascularization (TMR) relies on a CO2 or holmium:yttrium-aluminum-garnet (Ho:YAG) laser to create multiple transmural channels (1mm in diameter) through the myocardium. The initial concept was that these channels would serve as conduits for direct perfusion from the ventricle, but evidence suggests that the resultant angiogenesis is primarily responsible for the improved perfusion. A meta-analysis of seven randomized controlled trials comparing TMR to medical therapy for chronic angina have shown higher rates of angina improvement in the TMR but was not able to show a difference in mortality between the two groups.64
TMR is also being used as an adjunct to CABG in the treatment of extensive CAD that is not amenable to surgical revascularization alone. In a study looking at the benefits of TMR in addition to CABG, Allen et al concluded that TMR decreases angina burden when added to CABG in patients who cannot be revascularized by CABG alone.65 The current STS guidelines support the consideration of TMR in patients with ischemic myocardial territories that cannot be revascularized by PCI or CABG.66 Because of equivocal late results at most centers, this therapeutic strategy has not gained widespread acceptance.
Provocative investigations are being performed on the level of signaling molecules, gene therapy, stem cells, and tissue engineering to regenerate or replace damaged tissue in patients with ischemic heart disease. Growth factors, such as FGF and VEGF, are receiving focused attention due their ability to induce ingrowth of new vessels. Although concerns regarding systemic administration of these pleiotropic signaling molecules exist, early placebo-controlled clinical trials have shown some promising results with administration of these agents.67,68 Adenoviral transfection of diseased tissue with transgenes for these same growth factors has also been attempted with variable results.
Research in tissue engineering has been directed at creation of vascular conduits that are resistant to atherosclerosis. Stem cells have also been infused directly into the site of injury or in the generation of new tissue around a biodegradable scaffold. Despite their potential, these technologies are still in their infancy and significant progress will be needed before more widespread clinical adoption.
VALVULAR HEART DISEASE
The number of patients referred for the surgical management of valvular heart disease has increased substantially in recent years, with the percentage of isolated valve procedures performed in the United States increasing from 14% of all cardiac operations in 1996, to 22% in 2006.69 In 2012, valve proceduresrepresented over 50% of the cases performed at our institution. Although congenital and inherited etiologies represent important clinical entities, age-associated and acquired conditions still represent the primary causes of valvular heart disease, and are the focus of this section.
The most common screening method for valvular heart disease is cardiac auscultation, with murmurs classified based primarily on their timing in the cardiac cycle, but also on their configuration, location and radiation, pitch, intensity and duration (Table 21-7).70 Although some systolic murmurs are related to normal physiologic increases in blood flow, some may indicate cardiac disease, such as valvular aortic stenosis (AS), that are important to diagnose, even when asymptomatic. Diastolic and continuous murmurs, on the other hand, are frequently pathologic in nature. Dynamic cardiac auscultation provides further evidence as to the significance and origin of many murmurs (Table 21-8).70
| INTERVENTION | EFFECT |
|---|---|
| Respiration | Right-sided murmurs increase with inspiration. Left-sided murmurs increase with expiration. |
| Valsalva maneuver | Most murmurs decrease in length and intensity. The murmur of HCM becomes louder, and the murmur of MVP becomes louder and longer. |
| Exercise | Benign flow murmurs and murmurs caused by stenotic valves become louder with isotonic and isometric exercise. The murmurs of MR, VSD, and AI also increase with isometric exercise. |
| Positional changes | Most murmurs decrease with standing; the murmur of HCM becomes louder, and the murmur of MVP becomes louder and longer. Brisk squatting and passive leg raising increases most murmurs; the murmurs of HCM and MVP diminish. |
| Postventricular premature beat or atrial fibrillation | Benign flow murmurs and stenosis at the semilunar valves increase in intensity following a ventricular premature beat or a long cycle length in atrial fibrillation. Systolic murmurs of atrioventricular valve regurgitation do not change. |
| Pharmacologic interventions | The initial hypotensive phase following inhalation of amyl nitrate decreases the murmurs of MR, VSD, and AI, and increases the murmur of AS. The later tachycardic phase following inhalation of amyl nitrate increases right-sided murmurs and the murmur of MS. The response in MVP is biphasic (softer then louder than control). |
| Transient arterial occlusion | Transient external compression of the upper extremity increases the murmurs of MR, VSD, and AI. |
| CLINICAL SETTING | CLASS OF RECOMMENDATION | LEVEL OF EVIDENCE |
|---|---|---|
| • Echocardiography is recommended for asymptomatic patients with diastolic murmurs, continuous murmurs, holosystolic murmurs, late systolic murmurs, murmurs associated with ejection clicks, or murmurs that radiate to the neck or back. | I | C |
| • Echocardiography is recommended for patients with heart murmurs and symptoms or signs of heart failure, myocardial ischemia or infarction, syncope, thromboembolism, infective endocarditis, or other clinical evidence of structural heart disease. | I | C |
| • Echocardiography is recommended for asymptomatic patients who have grade 3 or louder midpeaking systolic murmurs. | I | C |
| • Echocardiography can be useful for the evaluation of asymptomatic patients with murmurs associated with other abnormal cardiac physical findings or murmurs associated with an abnormal electrocardiogram or chest x-ray. | IIa | C |
| • Echocardiography can be useful for patients whose symptoms and/or signs are likely noncardiac in origin but in whom a cardiac basis cannot be excluded by standard evaluation. | IIa | C |
| • Echocardiography is not recommended for patients who have a grade 2 or softer midsystolic murmur identified as innocent or functional by an experienced observer. | III – Harm | C |
Although auscultation may provide initial evidence to the existence of valvular disease, associated signs and symptoms may help narrow the diagnosis. Abnormalities in the splitting of the heart sounds and additional heart sounds should be noted, as should the presence of pulmonary rales. Peripheral pulses should be checked for abnormal intensity or timing, and the presence of a jugular venous wave should be documented. Additionally, symptoms of syncope, angina pectoris, heart failure, and peripheral thromboembolism are important and may help guide diagnosis and management.
Several imaging examinations are also available to aid in the diagnosis and classification of various valvular disorders. Electrocardiograms (EKGs) are widely available, and may provide information regarding ventricular hypertrophy, atrial enlargement, arrhythmias, conduction abnormalities, prior myocardial infarction, and evidence of active ischemia that would prompt further workup. Posteroanterior and lateral chest X-rays are also easy to obtain, and may yield information regarding cardiac chamber size, pulmonary blood flow, pulmonary and systemic venous pressure, and cardiac calcifications. The gold standard for the evaluation of valvular heart disease is transthoracic echocardiography (TTE).
Although helpful in the noninvasive evaluation of valve morphology and function, chamber size, wall thickness, ventricular function, pulmonary and hepatic vein flow, and pulmonary artery pressures, TTE may be unnecessary for some patients with asymptomatic cardiac murmurs. Current recommendations for evaluation via TTE are listed in Table 21-9.70 Specialized examinations based on the specific findings of TTE examinations are discussed as appropriate in the following sections.
| Mitral Stenosis | |||
| Indicator | Mild | Moderate | Severe |
| Mean gradient (mm Hg)* | <5 | 5–10 | >10 |
| Pulmonary artery systolic pressure (mm Hg) | <30 | 30–50 | >50 |
| Valve area (cm2) | >1.5 | 1.0–1.5 | <1.0 |
| Mitral Regurgitation | |||
| Qualitative | Mild | Moderate | Severe |
| Angiographic grade | 1+ | 2+ | 3+ |
| Color Doppler jet area | Small, central jet (<4 cm2 or <20% left atrial area) | More than mild criteria, but no severe criteria present | Vena contracta width >0.7 cm with large central jet (area >40% of left atrial area) or with a wall-impinging jet of any size, swirling in left atrium |
| Doppler vena contracta width (cm) | <0.3 | 0.3–0.69 | ≥0.7 |
| Quantitative (cath or echo) | |||
| Regurgitant volume (ml per beat) | <30 | 30–59 | ≥60 |
| Regurgitant fraction (%) | <30 | 30–49 | ≥50 |
| Regurgitant orifice area (cm2) | 0.2 | 0.2–0.39 | ≥0.4 |
| Additional essential criteria | |||
| Left atrial size | Enlarged | ||
| Left ventricular size | Enlarged | ||
Regardless of the etiology, valvular heart disease can produce a myriad of hemodynamic derangements. Left untreated, valvular stenosis and insufficiency can produce significant pressure and volume overload on the affected cardiac chamber, respectively, with mixed disease consequently causing mixed pathology. Although the heart can initially compensate for alterations in cardiac physiology, cardiac function eventually deteriorates, leading to heart failure, decreasing patient functional status, ventricular dysfunction, and eventually death. In order to optimize long-term survival, surgery is recommended in various forms of valvular heart disease, and in an increasing number of elderly and high-risk patients.
Although valve repair is increasingly indicated, especially in patients with aortic, mitral or tricuspid insufficiency, valve replacement may be necessary in certain patient populations. In some cases valve replacement can be accomplished with either mechanical or biological prostheses, and the choice of valve depends on many patient-specific factors such as age, health status, and desire for future pregnancy. Preexisting indications or contraindications to anticoagulation therapy also influence the choice of mechanical vs. tissue valve prosthesis.
Current options for mechanical valve replacement include tilting disc valves and bileaflet valves. Although mechanical valves are highly durable, they require permanent anticoagulation therapy to mitigate the otherwise high risk of valve thrombosis and thromboembolic sequelae.71 Due to the concordant risk of hemorrhagic complications, patient characteristics such as debility, lifestyle, and contraindications to systemic anticoagulation therapy may preclude mechanical valve replacement. Moreover, young women who are planning future pregnancies cannot take warfarin due to its teratogenic potential. Conversely, patients with other indications for systemic anticoagulation, such as other risk factors for thromboembolism (i.e., atrial fibrillation), or the existence of a mechanical prosthetic valve already in place in another position, may benefit from mechanical valve replacement. Additionally, patients with renal failure, on hemodialysis, or with hypercalcemia experience accelerated degeneration of bioprosthetic valves, and are thus, recommended to receive mechanical prostheses.72 In general, mechanical valve replacement is preferred in patients with expected long life spans who are acceptable candidates for anticoagulation therapy, in order to minimize reoperation and bleeding risks.
The potential to avoid the hazards of serious bleeding complications spurred the development of valve prostheses using biological materials, which obviate the need for systemic anticoagulation therapy. As tissue valves are naturally less thrombogenic, the attendant yearly risks of both thromboembolic and anticoagulation-related complications are considerably less than with mechanical valves.73 Consequently, tissue valve replacement is generally recommended for patients averse to systemic anticoagulation therapy, with potential concerns regarding compliance or follow-up while taking anticoagulant medications, and in the case of reoperation for a thrombosed mechanical valve. However, biological valves are more prone to degeneration, especially when implanted in the mitral position, in younger patients, and in patients in renal failure, on hemodialysis, or with hypercalcemia.73 Improved manufacturing methods have made currently available tissue valves more durable than previous versions, and valve replacement with a biological prosthesis is generally preferred in patients without other indications for anticoagulation therapy, who are >60 years of age for the aortic position, and >70 years of age for the mitral position.
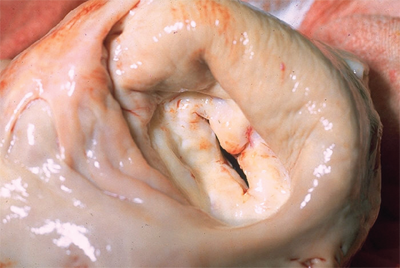
The first bileaflet valve was introduced in 1977. Bileaflet valves are comprised of two semicircular leaflets which open and close, creating one central and two peripheral orifices (Fig. 21-4). Bileaflet mechanical valves have demonstrated excellent flow characteristics, low risks of late valve-related complications, including valve failure, and are currently the most commonly implanted type of mechanical valve prosthesis in the world.72
Although mechanical valves necessitate systemic anticoagulation, careful monitoring of the International Normalized Ratio (INR) reduces the risk of thromboembolic events and hemorrhagic complications, and improves overall survival.74 Patients undergoing mechanical aortic valve replacement generally have a target INR of 2 to 3 times normal. Patients undergoing mechanical mitral valve replacement frequently have increased left atrial size, concomitant atrial fibrillation, and are at higher risk for thromboembolism that those undergoing mechanical aortic valve replacement, and are thus recommended to have a target INR 2.5 to 3.5 times normal. When managed appropriately, the yearly thromboembolic and bleeding risks in these patients are 1% to 2%, and 0.5% to 2%, respectively.
A xenograft valve is one implanted from another species, such as porcine xenograft valves, or manufactured from tissue such as bovine pericardium. A variety of xenograft tissue valves exist, and are primarily differentiated by the presence or absence of a mounting stent. Stented valves are the most commonly implanted, and the most popular valve in the United States is a stented bovine pericardial valve.
The more traditional stented valves are attached to a sewing ring, which decreases the technical complexity of valve replacement compared with stentless valves (Fig. 21-5). The chief disadvantage of stented tissue valves is a smaller effective orifice area, which increases the transvalvular gradient. This effect is most pronounced in patients with small prosthetic valve areas, specifically <0.85cm2 valve area per square meter body surface area, and may affect symptomatic improvement and the hemodynamic response to exercise following surgery.75
Stentless porcine xenograft valves were developed in order to minimize the limitations in flow characteristics seen in patients with small prosthetic valve areas, and have demonstrated an increase in effective valve area of approximately 10% over stented xenografts of equivalent size.72 They can result in improved hemodynamics, both at rest and with exercise.76 The absence of a stent and sewing ring both increases the technical complexity of valve replacement, and takes advantage of the biologic mobility of the aortic valve apparatus. Though results with stentless valves seem promising, long-term durability remains to be shown, and they have not been widely adopted due to the technical complexity associated with implantation.
Homograft valves from human cadavers, also known as allografts, have been used for aortic valve replacement since the technique was originally described over 50 years ago.77 Since that time, homografts have typically been used for aortic and pulmonary valve replacements, and have been successfully harvested from brain dead organ donors and the explanted hearts of heart transplant patients. Following harvest, these valves are sterilized using an antibiotic solution, and subsequently stored in fixative or cryopreserved.
Like other types of tissue valves, the risk of thromboembolic complications with homograft valves is low, and systemic anticoagulation therapy is not required. Additionally, the structure of homograft valves is naturally low-profile, allowing for larger effective valve orifices and lower postoperative transvalvular gradients compared with stented xenograft valves. Additionally, they have been shown to have some advantages in patients with endocarditis.78
The major shortcoming of homograft valves is their uncertain long-term durability in the face of significant tissue degeneration. Within one year of implantation, these valves undergo substantial loss of cellular components and subsequent structural compromise, which may ultimately lead to valve failure.79 Although enhanced preservation techniques significantly improve cellular viability, which approach the 15-year viability of xenograft valves, the availability of these techniques has limited the use of homograft tissue valves.
In 1967, Donald Ross described a procedure in which the diseased aortic valve is replaced using the patient’s native pulmonary valve as an autograft, which is in turn replaced with a homograft in the pulmonic position.80 The procedure results in minimal transvalvular gradients, and favorable left ventricular mechanics, both at rest and during exercise. Known as the Ross procedure, this operation is particularly beneficial in children, as the pulmonary trunk grows with the child and long-term anticoagulation is not required.81
The late results of the Ross procedure are discussed later in this chapter. In addition to potential concerns with durability, performance of the Ross procedure has also been limited by its technical complexity, and the increased surgical risk associated with double valve replacement.
Valve repair offers several advantages over valve replacement, due in large part to the preservation of the patient’s native valve and subvalvular apparatus. In the case of mitral valve (MV) surgery, preservation of the mitral apparatus has been shown to lead to better postoperative left ventricular function and survival.82,83 Additionally, as there is no implanted prosthesis, the patient avoids the risks of chronic anticoagulation, infection, thromboembolic complications, and prosthetic valve failure after surgery.
In the case of MV repair, freedom from reoperation and valve-related complications has been excellent in certain patient populations, even at 20-year follow-up.84 It has also been demonstrated that patients undergoing MV surgery with moderate functional tricuspid regurgitation (TR) do not experience increased perioperative complication rates when a concomitant tricuspid valve (TV) repair is performed.85 Midterm results in this group are encouraging, with greater than 98% freedom from reoperation reported by some groups at 5 years, suggesting further indications for valve repair.
Despite its advantages for the patient, valve repair is generally more technically demanding than valve replacement, and may occasionally fail. Both the suitability of the patient for valve repair and the skill and expertise of the surgeon performing the operation are important when considering valve repair in the individual patient.
MITRAL VALVE DISEASE
Acquired mitral stenosis (MS) is most often caused by rheumatic fever, with approximately 60% of patients with pure MS presenting with a positive clinical history of rheumatic heart disease.70 Rarely, other conditions can cause obstruction to filling of the left ventricle (LV), mimicking MS. Acquired causes of MV obstruction include left atrial myxoma, ball valve thrombus, mucopolysaccharidosis, previous chest radiation, and severe annular calcification.
Although rheumatic heart disease is associated with a transmural pancarditis, pathological fibrosis of the valves results primarily from the endocarditic process. The damage caused by endocardial inflammation and fibrosis is progressive, causing commissural fusion, subvalvular shortening of the chordae tendineae, and calcification of the valve and subvalvular apparatus.86 The resulting stenotic MV has a funnel-shaped apparatus, with a significantly narrowed orifice obliterated by interchordal and commissural fusion (Fig. 21-6). The degree of mitral stenosis should be determined preoperatively, as these pathological features may help determine the timing and type of intervention to perform.70
As the normal MV area of 4.0 to 5.0 cm2 is reduced by the rheumatic process, blood can flow from the left atrium to the left ventricle only if it is propelled by an ever-increasing pressure gradient. The diastolic transmitral gradient, which is a function of the square of the transvalvular flow rate and the diastolic filling period, is a fundamental expression of the severity of MS. When the valve area is reduced to <2.5 cm2, patients may begin to experience symptoms when the transmitral gradient is exacerbated by conditions that either increase transmitral flow or decrease diastolic filling time, such as exercise, emotional stress, infection, pregnancy, or atrial fibrillation with a rapid ventricular response.87 Symptoms may begin to occur at rest with the onset of moderate stenosis, defined as a cross-sectional area of 1.0 to 1.5 cm2, and any physical exertion is typically limited by the time the MV area is <0.8 to 1.0 cm2 (Table 21-10).70
| CLINICAL SETTING | CLASS OF RECOMMENDATION | LEVEL OF EVIDENCE |
|---|---|---|
| Balloon Valvotomy for Mitral Stenosis | ||
• Symptomatic patients (NYHA II, III, IV) with moderate or severe MS and favorable valve morphology, without left atrial thrombus or moderate to severe MR • Asymptomatic patients with moderate or severe MS, favorable valve morphology, and pulmonary hypertension (PASP >50 mm Hg at rest, >60 mm Hg with exercise), without left atrial thrombus or moderate to severe MR • Symptomatic patients (NYHA III, IV) with moderate or severe MS and favorable valve morphology, who are high risk or not candidates for surgery • Asymptomatic patients with moderate or severe MS, favorable valve morphology, and new onset atrial fibrillation, without left atrial thrombus or moderate to severe MR • Symptomatic patients (NYHA II, III, IV) with MV area >1.5 cm2 if there is evidence of hemodynamically significant MS (PASP >60 mm Hg, PAWP ≥25 mm Hg, mean MV gradient >15 mm Hg during exercise) • Symptomatic patients (NYHA III, IV) with moderate or severe MS and favorable valve morphology, as an alternative to surgery • Patients with mild MS • Patients with moderate to severe MR or left atrial thrombus | I I IIa IIb IIb IIb III – Harm III – Harm | A C C C C C C C |
| Surgery for Mitral Stenosis* | ||
• Symptomatic patients (NYHA III, IV) with moderate or severe MS when: Balloon valvotomy is unavailable Balloon valvotomy is contraindicated due to thrombus or MR Valve morphology is not favorable for balloon valvotomy • Symptomatic patients with moderate to severe MS who also have moderate to severe MR • Mildly symptomatic patients (NYHA I, II) with severe MS and severe pulmonary hypertension (PASP >60 mm Hg) • Asymptomatic patients with moderate or severe MS and recurrent embolic events while receiving adequate anticoagulation, when the likelihood of successful MVr is high • MVr in the setting of mild MS • Closured commissurotomy in the setting of MVr; open commissurotomy should be performed | I I IIa IIb III – Harm III – Harm | B C C C C C |
| Surgery for Mitral Regurgitation* | ||
• Symptomatic patients with acute severe MR • Symptomatic patients (NYHA II, III, IV) with chronic severe MR without LV dysfunction (LVEF <0.30) and/or end-systolic dimension >55 mm • Asymptomatic patients with chronic severe MR and mild to moderate LV dysfunction (LVEF 0.30–0.60) and/or end-systolic dimension ≥40 mm • Asymptomatic patients with chronic severe MR and preserved LV function (LVEF >0.60, end-systolic dimension <40 mm), when the likelihood of successful MVr is >90% • Asymptomatic patients with chronic severe MR, preserved LV function, and 1) New onset atrial fibrillation, 2) Pulmonary hypertension (PASP >50 mm Hg at rest, >60 mm Hg with exercise) • Symptomatic patients (NYHA III, IV) with chronic severe MR due to a primary abnormality of the mitral apparatus and severe LV dysfunction (LVEF <0.30, end-systolic dimension >55 mm), when the likelihood of successful MVr is high • Symptomatic patients (NYHA III, IV) with chronic severe MR secondary to severe LV dysfunction (LVEF <0.30) who remain symptomatic despite optimal medical management for heart failure, including biventricular pacing • Asymptomatic patients with MR and preserved LV function (LVEF >0.60, end-systolic dimension <40 mm), when the likelihood of successful repair is low • Isolated MV surgery in the setting of mild or moderate MR |
||