KEY POINTS
There are important anatomic differences in the rectus sheath structures above and below the arcuate line. The laminae of the internal oblique, which contribute to both the anterior and (along with the transversus abdominis) posterior rectus above the arcuate line, only contribute to the anterior sheath below the arcuate line. There is no aponeurotic posterior covering on the lower portion of the rectus muscles.
Rectus diastasis is associated with abdominal wall bulging consequent to separation of the rectus abdominis muscles in the midline. It does not represent a hernia, and surgical interventions for this condition are of questionable, if any, clinical benefit.
When resection of abdominal wall desmoid tumors is undertaken, it must be recognized that failure to achieve negative margins is associated with an extremely high risk of local recurrence of the tumor.
Primary repair of ventral incisional hernias is associated with unacceptably high failure rates, and repair using other approaches, such as use of prosthetic mesh, is preferred.
The addition of the closed videoscopic technique to components separation procedures has been associated with a significant decrease in the incidence of local wound complications.
Potential benefits of laparoscopic incisional hernia repairs compared to open repairs with mesh include shorter hospitalization, lower risk of wound complications, and better abdominal wall function. A lower recurrence rate benefit remains controversial.
Surgical treatment of sclerosing mesenteritis is most often undertaken to confirm diagnosis and to rule out neoplasm as the cause of a mesenteric mass. Resection possibilities are limited by the extensiveness of the process as well as by the questionable benefit in most cases.
Potential surgical interventions in retroperitoneal fibrosis include operative biopsy to rule out neoplasm, ureteral stent placement, open or laparoscopic ureterolysis, and endovascular interventions for iliocaval occlusion.
ABDOMINAL WALL
The abdominal wall provides structure, protection, and support for abdominal and retroperitoneal structures and is defined superiorly by the costal margins, inferiorly by the pelvic ring, and posteriorly by the vertebral column. Knowledge of its specific anatomic features is required for management of abdominal wall diseases or during entry into the peritoneal cavity.
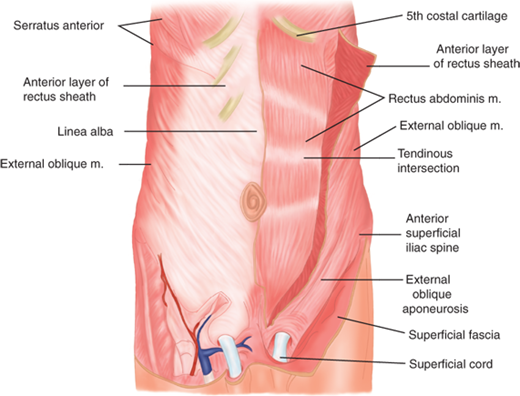
The abdominal wall is an anatomically complex, layered structure with segmentally derived blood supply and innervation (Fig. 35-1). It is mesodermal in origin and develops as bilateral migrating sheets, which originate in the paravertebral region and envelop the future abdominal area. The leading edges of these structures develop into the rectus abdominis muscles, which eventually meet in the anterior midline. The rectus abdominis is longitudinally oriented and encased within an aponeurotic sheath, the layers of which are fused in the midline at the linea alba. The rectus insertions are on the pubic bones inferiorly and on the fifth and sixth ribs, as well as the seventh costal cartilages and the xiphoid process superiorly. The lateral border of the rectus muscles has a curved shape identifiable as the surface landmark, the linea semilunaris. Three tendinous intersections cross the rectus muscles at the level of the xiphoid process, umbilicus, and about halfway between the xiphoid process and the umbilicus (see Fig. 35-1).
Figure 35-1.
Anterior abdominal wall. Linea alba is the midline aponeurotic demarcation between the bellies of the rectus abdominis muscles. The rectus abdominis muscle and its tendinous intersections on the left are shown deep to the reflected anterior rectus sheath. (Reproduced with permission from Moore KL, Dalley AF, eds. Clinically Oriented Anatomy. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 1999:181.)
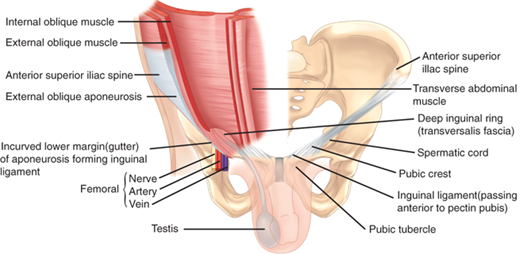
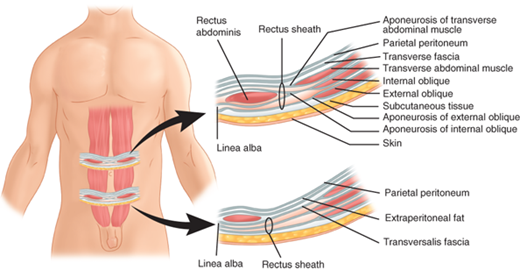
Lateral to the rectus sheath are three muscular layers with oblique fiber orientations relative to one another (Fig. 35-2). These layers are derived from laterally migrating mesodermal tissues during the sixth to seventh week of fetal development. The external oblique muscle runs inferiorly and medially, arising from the margins of the lowest eight ribs and costal cartilages. The external oblique muscles originate on the latissimus dorsi and serratus anterior muscles, as well as on the iliac crest. Medially, the external obliques form a tendinous aponeurosis, which is contiguous with the anterior rectus sheath. The inguinal ligament is the inferior-most edge of the external oblique aponeurosis, reflected posteriorly in the area between the anterior superior iliac spine and pubic tubercle. The internal oblique muscle lies deep to the external oblique and arises from the lateral aspect of the inguinal ligament, the iliac crest, and the thoracolumbar fascia. Its fibers course superiorly and medially and form a tendinous aponeurosis that contributes components to both the anterior and posterior rectus sheath. The lower medial and inferior-most fibers of the internal oblique may fuse with the lower fibers of the transversus abdominis muscle (the conjoined area). The inferior-most fibers of the internal oblique muscle are contiguous with the cremasteric muscle in the inguinal canal. These relationships are of critical significance in the management of inguinal hernias. The transversus abdominis muscle is the deepest of the three lateral muscles and runs transversely from the lowest six ribs, the lumbosacral fascia, and the iliac crest, to the lateral border of the rectus abdominis. The arcuate line (semicircular line of Douglas) lies roughly at the level of the anterior superior iliac spines (Fig. 35-3). Above the arcuate line, the anterior rectus sheath is formed by the external oblique aponeurosis and the external lamina of the internal oblique aponeurosis, whereas the posterior rectus sheath is formed by the internal lamina of the internal oblique aponeurosis and the transversus abdominis aponeurosis. Below the arcuate line, the anterior rectus sheath is formed by the external oblique aponeurosis, the laminae of the internal oblique aponeurosis, and the transversus abdominis aponeurosis. There is no aponeurotic posterior covering of this lower portion of the rectus muscles, although the endoabdominal, or transversalis, fascia provides contiguous coverage of the posterior aspect of the abdominal above and below the arcuate line.
Figure 35-2.
The three muscular layers of the abdominal wall lateral to the rectus abdominis are the external oblique, internal oblique, and transversus abdominis muscles, shown here on the low abdomen, where the lower margin of the external oblique reflects posteriorly as the inguinal ligament. (Reproduced with permission from Moore KL, Dalley AF, eds. Clinically Oriented Anatomy. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 1999:181.)
Figure 35-3.
Cross-sectional anatomy of the abdominal wall above and below the arcuate line of Douglas. The lower right abdominal wall segment shows clearly the absence of an aponeurotic covering of the posterior aspect of the rectus abdominis muscle inferior to the arcuate line. Superior to the arcuate line, there are both internal oblique and transversus abdominis aponeurotic contributions to the posterior rectus sheath. (Reproduced with permission from Moore KL, Dalley AF, eds. Clinically Oriented Anatomy. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 1999:185.)
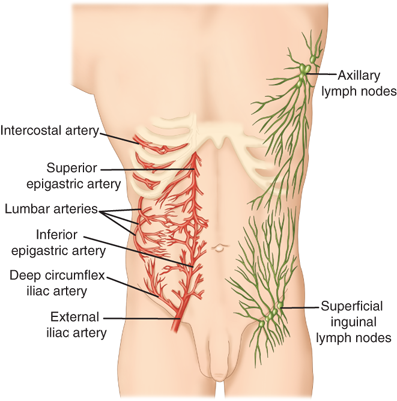
The blood supply to the muscles of the anterior abdominal wall is derived mainly from the superior and inferior epigastric arteries (Fig. 35-4). The superior epigastric artery arises from the internal thoracic artery, while the inferior epigastric artery arises from the external iliac artery. A collateral network of branches of the subcostal and lumbar arteries also contributes the abdominal wall blood supply. The lymphatic drainage of the abdominal wall is predominantly to the major nodal basins in the superficial inguinal and axillary areas.
Anterior abdominal wall innervation is segmental. Motor nerves to the rectus, oblique, and transversus abdominis muscles run from the anterior rami of spinal nerves at the T6 to T12 levels. The overlying skin is innervated by afferent branches of the T4 through L1 nerve roots, with T10 nerve roots providing sensation around the umbilicus (Fig. 35-5).
Figure 35-4.
The superior and inferior epigastric arteries form an anastomosing network of vessels in and around the rectus sheath, with collateralization to subcostal and lumbar vessels situated more laterally on the abdominal wall. Lymphatic drainage is via axillary or inguinal nodal basins. (Reproduced with permission from Moore KL, Dalley AF, eds. Clinically Oriented Anatomy. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 1999:188.)
The rectus muscles, external obliques, and internal obliques work as a unit to flex the trunk anteriorly or laterally. Trunk rotation is achieved by simultaneous contraction of a unilateral external oblique and the contralateral internal oblique (e.g., rotation to the right is produced by contraction of the left external oblique muscle and the right internal oblique). All of the truncal muscles are involved in raising intra-abdominal pressure. Abdominal musculature contraction that occurs when the diaphragm is relaxed will result in expiration of air from the lungs, or a cough if this contraction is forceful. If the diaphragm is contracted when the abdominal musculature is contracted (Valsalva maneuver), the increased abdominal pressure aids in processes such as micturition, defecation, and childbirth.
Surgeons must deal with the abdominal wall to access pre-, intra-, and retroperitoneal sites. This begs practical questions of where and how to make incisions. Incisions for open surgery are generally located in proximity to the principal operative targets. Laparoscopic port site incisions might be remote from the site of interest and are carefully planned based on the instrument approach angles and working distances both to the operative site and between ports. Orientation of any incision may be determined based on expected quality of exposure, closure considerations including cosmesis, avoidance of previous incision sites, and surgeon preference. In general, incisions for open peritoneal access can be longitudinal (in or off the midline), transverse (lateral to or crossing midline), or oblique (directed either upward or downward toward the flank) (Fig. 35-6). Modifications are numerous and can consist of various extensions to optimize exposure in specific clinical situations. Midline incisions are used for the majority of nonlaparoscopic procedures on the gastrointestinal tract. Incising the fused midline aponeurotic tissue of the linea alba is simple and does not injure skeletal muscle. Paramedian incisions through the rectus abdominis sheath structures have largely been abandoned in favor of midline or nonlongitudinal incisions. Incisions lateral to the midline made with transverse or oblique orientations can either divide the successive muscular layers or bluntly separate the fibers. This latter muscle-splitting approach, exemplified by the classic McBurney incision for appendectomy, may be less destructive to tissue but offers more limited exposure. Subcostal incisions on the right (Kocher incision for cholecystectomy) or left (for splenectomy) are archetypal muscle-dividing incisions that result in transection of intervening musculoaponeurotic tissues, including a portion of the rectus abdominis. These incisions are closed in two layers, the more superficial one incorporating the anterior aponeurotic sheath of the rectus medially, transitioning to external oblique muscle and aponeurosis laterally. The posterior, deeper layer consists of internal oblique and transversus abdominis muscle. Similar anatomic considerations are guide closure of transversely oriented muscle-dividing incisions. The Pfannenstiel incision, used commonly for pelvic procedures, is distinguished by transverse skin and anterior rectus sheath incisions, followed by rectus muscle retraction and longitudinal incision of the peritoneum. Irrespective of the incision type, suture apposition of abdominal wall tissues during closure is accomplished without significant tension and with great precision.
Figure 35-6.
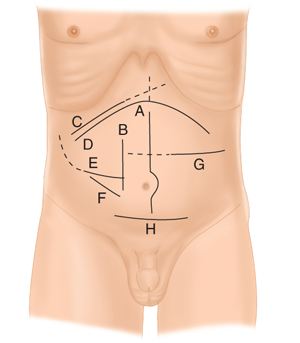
Various anterior abdominal wall incisions for exposure of peritoneal structures. A. Midline incision; B. paramedian incision; C. right subcostal incision and “saber slash” extension to costal margin (dashed line); D. bilateral subcostal (also buckethandle, chevron, gable) incision and “Mercedes Benz” extension (dashed line); E. Rocky-Davis incision and Weir extension (dashed line); F. McBurney incision; G. transverse incision and extension across midline (dashed line); and H. Pfannenstiel incision.
Abdominal incisions can lead to short- and long-term complications and patient disability. The question of how large an incision ought to be has no simple answer. In general, it is prudent to make incisions no larger than necessary to safely accomplish the operative goals. Laparoscopic and other minimally invasive surgical methods owe their development in large part to the belief that minimizing surgical injury to the abdominal wall is of significant benefit to the patient. For open surgery, a variety of devices are available to retract the abdominal wall and facilitate peritoneal exposure without subjecting the patient to excessively large incisions or surgical personnel to exhausting retraction tasks (Fig. 35-7). Examples include the Bookwalter™, Omni-Tract¯, and Thompson retractors.
The abdominal wall layers begin to form within in first weeks following conception. In early embryonic development, there is a large central defect through which pass the vitelline (omphalomesenteric) duct and allantois. The vitelline duct connects the embryonic and fetal midgut to the yolk sac. During the sixth week of development, the abdominal contents grow too large for the abdominal wall to completely contain them, and the embryonic midgut herniates into the umbilical cord. While outside the developing abdomen, it undergoes a 270-degree counterclockwise rotation on the developing mesentery. At the end of the twelfth week, it returns to the abdominal cavity. Defects in abdominal wall closure may lead to omphalocele or gastroschisis. In omphalocele, viscera protrude through an open umbilical ring and are covered by a sac derived from the amnion. In gastroschisis, the viscera protrude through a defect lateral to the umbilicus and no sac is present.
During the third trimester, the vitelline duct regresses. Persistence of a vitelline duct remnant on the ileal border results in a Meckel’s diverticulum. Complete failure of the vitelline duct to regress results in a vitelline duct fistula, which is associated with drainage of small intestinal contents from the umbilicus. If both the intestinal and umbilical ends of the vitelline duct regress into fibrous cords, a central vitelline duct (omphalomesenteric) cyst may occur. Persistent vitelline duct remnants between the gastrointestinal tract and the anterior abdominal wall may be associated with small intestinal volvulus in neonates. When diagnosed, vitelline duct fistulas and cysts should be excised along with any accompanying fibrous cord.
The urachus is a fibromuscular tubular extension of the allantois that develops with the descent of the bladder to its pelvic position. Persistence of urachal remnants can result in cysts as well as fistulas to the urinary bladder with drainage of urine from the umbilicus. These are treated by urachal excision and closure of any bladder defect that may be present.
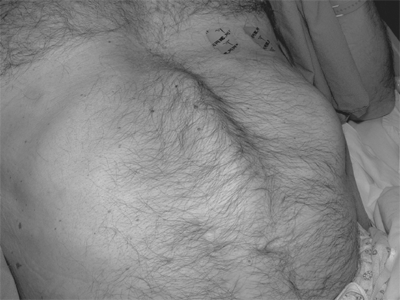
Rectus abdominis diastasis (or diastasis recti) results from a separation of the two rectus abdominis muscle pillars. This results in the characteristic bulging of the abdominal wall in the epigastrium that is sometimes mistaken for a ventral hernia despite the fact that the midline aponeurosis is intact and no hernia defect is present. Diastasis may be congenital, as a result of a more lateral insertion of the rectus muscles to the ribs and costochondral junctions, but is more typically an acquired condition with advancing age, obesity, or following pregnancy. In the postpartum setting, rectus diastasis tends to occur in women of advanced maternal age, after multiple or twin pregnancies, or in women who deliver high-birth-weight infants. Diastasis is usually easily identified on physical examination (Fig. 35-8). Computed tomography (CT) scanning can provide an accurate measure of the distance between the rectus pillars and will differentiate rectus diastasis from a true ventral hernia if clarification is required. Surgical correction of rectus diastasis by plication of the broad midline aponeurosis has been described for cosmetic indications and for disability of abdominal wall muscular function. However, these approaches introduce the risk of an actual ventral hernia and are of questionable value in addressing any actual pathology.
Hemorrhage from the network of collateralizing vessels within the rectus sheath and muscles can result in a rectus sheath hematoma. Although a history of trauma might be elicited, other less obvious events including sudden contraction of the rectus muscles with coughing, sneezing, or any vigorous physical activity may also cause this condition. Spontaneous rectus sheath hematomas occur most frequently in the elderly and in those on anticoagulation therapy. Patients frequently describe the sudden onset of unilateral abdominal pain that may be confused with lateralized peritoneal disorders such as appendicitis.
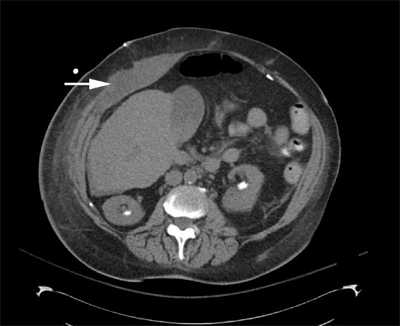
History and physical examination alone may be diagnostic. Pain typically increases with contraction of the rectus muscles, and a tender mass may be palpated. The ability to appreciate an intra-abdominal mass is ordinarily degraded with contraction of the rectus muscles. Fothergill’s sign is a palpable abdominal mass that remains unchanged with contraction of the rectus muscles and is classically associated with rectus hematoma. A hemoglobin/hematocrit level and coagulation studies should be obtained. Both ultrasonography and CT (Fig. 35-9) can provide confirmatory imaging information and exclude other disorders.
Specific treatment depends on the severity of the hemorrhage. Small, unilateral, and stable hematomas may be observed without hospitalization. Bilateral or large hematomas will likely require hospitalization, as well as potential resuscitation. Transfusion or coagulation factor replacement may be indicated in some situations. Angiographic embolization is required infrequently, but may be necessary if hematoma enlargement, free bleeding, or clinical deterioration occurs. Surgical therapy is used in the rare situations of failed angiographic treatment or hemodynamic instability that precludes any other options. The operative goals are evacuation of the hematoma and ligation of any bleeding vessel identified. Mortality in this condition is rare, but has been reported in patients requiring surgical treatment and in the elderly.
Desmoid tumors of the abdominal wall are fibrous neoplasms originating from the musculoaponeurotic structures of the anterior abdomen. They are also referred to collectively as aggressive fibrosis, a term that describes their aggressive and infiltrative local behavior. They do not have metastatic potential, and although there is marked cellularity in biopsy specimens, there are no specific histologic characteristics that suggest malignancy, per se. Desmoid tumors of the abdominal wall have a slight female predominance and occur either sporadically or in the setting of familial adenomatous polyposis (FAP), with the greatest risk incurred in Gardner’s syndrome. As many as 10% to 15% of FAP patients develop desmoid tumors of the abdominal wall, abdomen, or retroperitoneum, and this condition can account for patient mortality from complications of aggressive local growth. In non-FAP settings, abdominal wall desmoids occur most frequently in postpartum women or in surgical scars. Radical resection with frozen section margins and immediate mesh reconstruction of any consequent abdominal wall defect is the most commonly recommended treatment. Involvement of margins is associated with recurrence rates as high as 80%. Extensive infiltration and involvement of peritoneal structures frequently makes desmoid resection technically unfeasible. Medical treatment with an antineoplastic agent such as doxorubicin, dacarbazine, or carboplatin can produce remission for variable periods in up to 50% of patients, although the prognosis of advanced desmoids, particularly in FAP, is poor, with a 5-year mortality rate as high as 50% reported. Combined medical treatments and the addition of imatinib have been used with some success in small numbers of patients; radiation therapy has been used in both adjuvant and palliative roles with high response rates.
The abdominal wall may be the site of various benign neoplasms including lipomas and neurofibromas. Surgical treatment is not always mandatory, but local excision is recommended for symptomatic or enlarging lesions. Primary abdominal wall malignancies are exceedingly rare and include subtypes of sarcomas (leiomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma, liposarcoma, and rhabdomyosarcoma), dermatofibrosarcoma protuberans, schwannoma, and melanoma. Magnetic resonance imaging (MRI) or CT is used for tumor staging, and chest CT should be included to rule out pulmonary metastases. These studies define the extent of the tumor and involvement of contiguous structures in anticipation of surgical treatment. Prior to surgery for abdominal wall sarcomas, a core needle biopsy is generally obtained (with image guidance if needed). Once the diagnosis is established, treatment consists of resection with tumor-free margins, applying the same general principles used for extremity sarcoma resection. Meticulous dissection avoiding violation of tumor capsule and maintaining margins greater than 2 cm, if possible, are essential considerations. Extensive resection may leave a considerable abdominal wall defect that will have to be reconstructed. Immediate reconstruction with mesh and/or wound coverage with rotational or free myocutaneous flaps are the best options if primary closure is not feasible. Although these tumors are frequently described as radiation- and chemotherapy-resistant, both modalities have been used in advanced cases in both adjuvant and palliative settings. The very limited experience in these rare conditions makes commentary on the effectiveness of these therapies difficult.
Abdominal wall resection may also be required with contiguous involvement of gastrointestinal or gynecologic malignancies. Primary closure may be feasible, but prosthetic mesh use (even in the setting of bowel resection), absorbable or biologic mesh reinforcement, and myocutaneous flap reconstruction are also options.
Hernias of the anterior abdominal wall, or ventral hernias, represent defects in the parietal abdominal wall fascia and muscle through which intra-abdominal or preperitoneal contents can protrude. Ventral hernias may be congenital or acquired. Acquired hernias may develop via slow architectural deterioration of the musculoaponeurotic tissues, or they may develop from failed healing of an anterior abdominal wall incision (incisional hernia). The most common finding is a mass or bulge, which may increase in size with Valsalva. Ventral hernias may be asymptomatic or cause a considerable degree of discomfort and will generally enlarge over time. Physical examination reveals a bulge on the anterior abdominal wall that may reduce spontaneously, with recumbency, or with manual pressure. A hernia that cannot be reduced is described as incarcerated and generally requires surgical correction. Incarceration of an intestinal segment may be accompanied by nausea, vomiting, and significant pain, and is a true surgical emergency. If the blood supply to the incarcerated bowel is compromised, the hernia is described as strangulated, and the localized ischemia may lead to infarction and perforation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree