Key Points
Disease summary:
Nonalcoholic fatty liver disease (NAFLD) refers to a spectrum of conditions involving excess fat accumulation in the liver. The term encompasses conditions ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), which is defined by histologic findings of steatosis, inflammation, ballooned hepatocytes, Mallory-Denk bodies, and varying degrees of fibrosis. Simple steatosis is thought to have a benign prognosis, whereas a subset of individuals with NASH progress to end-stage complications of cirrhosis and hepatocellular carcinoma. The pathogenesis of NAFLD has not been clearly defined; however, there is a strong association with metabolic syndrome and insulin resistance.
Differential diagnosis:
Alcoholic liver disease, viral hepatitis, autoimmune hepatitis, hemochromatosis, Wilson disease, medication-induced fatty liver disease (amiodarone, tamoxifen, valproic acid, TPN).
Monogenic forms:
There is no single gene known to cause NAFLD. However, there are rare monogenic inherited disorders of lipid metabolism, insulin signaling, and mitochondrial function that result in hepatic steatosis. These disorders usually have other phenotypic manifestations which dominate over hepatic steatosis.
Family history:
Small studies in kindreds demonstrate a higher prevalence of NAFLD among first-degree relatives of patients. One study demonstrated a 59% prevalence of fatty liver in siblings and a 78% prevalence of fatty liver in parents of children with NAFLD. About 18% of patients with NASH have an affected first-degree relative.
Twin studies:
One study of monozygotic twins discordant for obesity demonstrated intrapair differences in liver fat that correlated with acquired obesity, suggesting the presence of strong nongenetic determinants of hepatic steatosis.
Environmental factors:
Western diets containing increased amounts of high fructose corn syrup, saturated fats, and trans fats have been implicated in rising obesity trends and increasing incidence of NAFLD.
Genome-wide associations:
The strongest association has been with a single-nucleotide polymorphism (SNP) variant in the PNPLA3 gene (also called “adiponutrin”). Other SNPs have been described in patients with NAFLD (Table 75-1). Testing for gene variants is not yet clinically validated for diagnosis or management of NAFLD.
Pharmacogenomics:
There are no known genetic predictors of response to pharmacotherapy.
| Candidate Gene (Chromosome Location) | Associated Variant [effect on protein] (assayed by Affymetrix/Illumina) | Relative Risk | Frequency of Risk Allele | Putative Functional Significance | Associated Disease Phenotype |
|---|---|---|---|---|---|
PNPLA3 Patatin-like phospholipase 3 (22q13.31) | Met148 [nonsynonymous amino acid substitution 1148M] rs738409 | Odds ratio 3.26 | 0.49 (Hispanics) 0.23 (European Americans) 0.17 (African Americans) | Loss-of-function mutation leading to impaired hydrolysis of triglycerides | Hepatic triglyceride accumulation (on 1H MRS) Increased histologic severity (steatosis, inflammation, ballooning, and fibrosis) Increased histologic severity in pediatric NAFLD |
| GCKR(2p23.3-p23.2) | P-446L rs780094 | 0.47 | Regulation of glucose storage/disposal, provides substrates for de novo lipogenesis | Hepatic steatosis (on CT) Histologic NAFLD Increased LDL Increased triglycerides | |
| NCAN(19p12) | P-91S rs2228603 | 0.12 | Adhesion molecule | Hepatic steatosis (on CT) Histologic NAFLD Decreased LDL Decreased triglycerides | |
| LYPLAL1(1) | rs12137855 | 0.83 | Impaired triglyceride breakdown | Hepatic steatosis (on CT) Histologic NAFLD Decreased fasting glucose | |
| APOC3 Apolipoprotein C3(11q23.1-q23.2) | rs2854116 (T-455C) rs2854117 (C-482T) | Increased apolipoprotein C3 concentration, inhibition of lipoprotein lipase, impaired plasma triglyceride clearance | Hepatic steatosis, hypertriglyceridemia, insulin resistance | ||
ENPP1 Ectoenzyme nucleotide pyrophosphate phosphodiesterase 1/PC-1 (6q22-q23) | Lys121Gln | Modulation of insulin sensitivity | Fibrosis stage >1 in NAFLD | ||
IRS-1 Insulin receptor substrate-1 (2q36) | Gly972Arg | Fibrosis stage >1 in NAFLD | |||
TNF-α (6p21.3) | TNFA 238 | Inflammation, insulin resistance | Increased insulin resistance NASH | ||
MTTP Microsomal triglyceride transfer protein (4q24) | −493G | Lipid metabolism | Hepatic steatosis |
Diagnostic Criteria and Clinical Characteristics
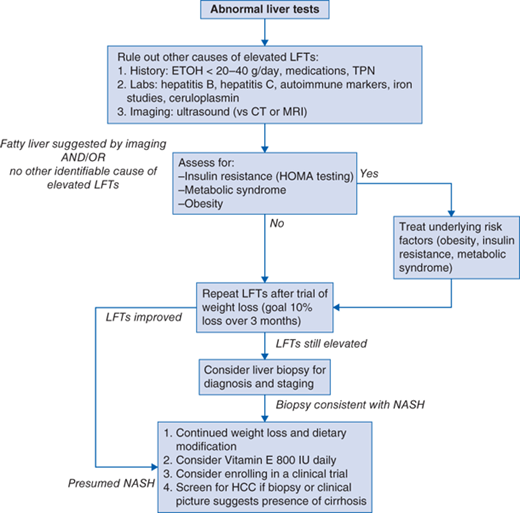
Diagnostic evaluation should include at least one of the following (Fig. 75-1 algorithm):
Exclusion of other causes of abnormal liver tests: hepatitis B, hepatitis C, autoimmune hepatitis, hemochromatosis, Wilson disease, drug- or alcohol-induced liver injury.
Careful history taking to rule out alcoholic liver disease (guidelines suggest a threshold of <20 g/d in women and <40 g/d in men although these limits are not well established).
Rule out other secondary causes of steatosis such as medications (valproic acid, amiodarone, tamoxifen, total parenteral nutrition).
Anthropometric measurements (body mass index [BMI], waist circumference).
Assessment for presence of metabolic syndrome (hypertension, increased waist circumference, dyslipidemia, diabetes).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree