Key Points
Clinical utility of thiopurine methyltransferase analysis:
Various guidelines recommend pre-thiopurine therapy thiopurine methyltransferase (TPMT) assessment in managing chronic inflammatory conditions.
There is insufficient evidence base to recommend pretherapy TPMT assessment.
Decreased or absent TPMT activity is associated with increased risk of myelotoxicity and leukopenia in patients treated with thiopurine medications (azathioprine [AZA] or 6-mercaptopurine [6-MP]).
Decreased or absent TPMT activity is not associated with hepatitis or pancreatitis in patients treated with thiopurine medications (AZA or 6-MP).
Reduced or absent TPMT activity does not place patients at risk of disease.
Medication(s) affected:
Thiopurine drugs AZA and 6-MP. Limited effects on 6-thioguanine (6-TG), as TPMT is not the major metabolic enzyme.
Drug-related adverse events:
Thiopurine treatment may be associated with anemia, myelotoxicity and leukopenia, hepatitis, and pancreatitis.
TPMT deficiency:
The presence of mutant TPMT alleles or decreased TPMT enzymatic activity increases the risk of thiopurine treatment-related myelotoxicity and leukopenia.
Non-TPMT–associated myelosuppression:
Normal TPMT testing does not rule out all the risk of myelotoxicity for patients on thiopurine medications.
Analytic methods:
Genotyping—most common mutant alleles (TPMT*2, TPMT*3A, TPMT*3B, and TPMT*3C) are routinely available through reference laboratories.
Enzymatic analysis—less commonly available, but is the preferred method as it will also detect rare mutations not identified by genetic screening. Recent blood transfusions (within 90 days) can produce incorrect results.
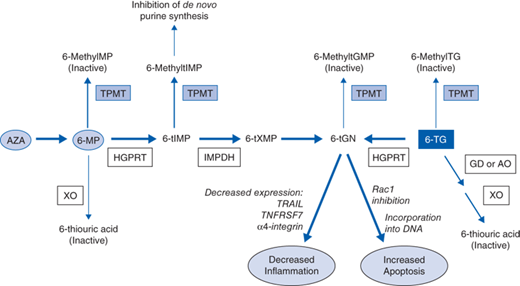
Thiopurine Drugs and Metabolic Pathway
AZA, 6-MP, and 6-TG are thiopurine-based prodrugs that have no intrinsic biologic activity, and require extensive metabolism for activity (Fig. 5-1). After oral administration of AZA or 6-MP, between 27% and 83% is available as biologically active metabolites. AZA is often used clinically, as it is more stable and soluble than 6-MP. AZA doses are higher because the molecular weight of 6-MP is 55% of that of AZA.
In the gut, approximately 90% of AZA is converted to 6-MP, a thiopurine analogue of the purine base hypoxanthine, by cleavage of the imidazolyl moiety which is thought to be catalyzed through the action of glutathione transferase. 6-MP is then enzymatically converted to its active metabolite, deoxy-6-thioguanosine 5’ triphosphate (6-tGN), through successive enzymatic conversions by hypoxanthine-guanine phosphoribosyl transferase (HGPRT) and inosine monophosphate dehydrogenase (IMPDH). Inactivation of 6-MP (and hence AZA) occurs primarily through S-methylation by thiopurine S-methyltransferase (TPMT), and to a minor degree by catabolism, to thiouric acid by xanthine oxidase (XO). 6-TG is converted to its active metabolite (6-tGN) in a single step involving HGPRT, while inactivation occurs through two pathways. The major metabolic pathway involves guanine deaminase (GD) and aldehyde oxidase (AO) to form inactive 6-thiouric acid. Metabolism by TPMT, to form inactive 6-methyl-TG, is a minor contributor to drug inactivation. TPMT also plays a minor role in directly methylating and inactivating 6-tGN.
Incorporation of 6-tGN into DNA triggers cell-cycle arrest and apoptosis through the mismatch repair pathway. Until recently, this was considered the primary mechanism of action. However, recent evidence has suggested other mechanisms of immunosuppression not directly related to 6-tGN incorporation into DNA. Metabolism by TPMT of 6-thiomercaptopurine (6-tIMP), an intermediate metabolite, to produce 6-methyl–tIMP has been shown to inhibit de novo purine synthesis in lymphocytes, which likely contributes to the immunosuppressive effects of thiopurines. Furthermore, accumulation of 6-tGN in lymphocytes has been demonstrated to decrease the expression of TRAIL, TNFRS7, and α-4 integrin, effectively decreasing inflammation. Thiopurine drugs have also been shown to induce apoptosis in T cells through modulation of Rac1 activation on CD28 costimulation. Rac1 is a GTPase upstream of MEK, NF-κB, and bcl-xL. On binding of 6-thio-GTP with Rac1, activation of its downstream mediators is blocked, inducing apoptosis.
TPMT represents the predominant inactivation pathway of thiopurines in hematopoietic cells. Thiopurine-based drugs have been associated with various toxic adverse events, including myelosuppression, hepatotoxicity, pancreatitis, and flu-like symptoms, among others. One of the most serious dose-dependent reactions is myelosuppression, which is believed to be caused by increased 6-tGN levels (the active metabolite), either due to overdosing or a decreased rate of thiopurine metabolism due to impaired activity of the TPMT enzyme.
TPMT Polymorphisms
The TPMT gene is located on chromosome 6 at 6p22.3. It is approximately 27 kb in size and contains nine exons. A nonfunctional TPMT pseudogene has also been identified on chromosome 18 at 18q21.1. TPMT is widely expressed in many tissues, but TPMT expression in lymphocytes, red blood cells, and bone marrow is most relevant clinically for immunosuppression by thiopurine drugs. To date, at least 30 variant (or mutant) alleles of TPMT have been identified, the majority of which have been associated with lower TPMT enzymatic activity or protein expression (Table 5-1).
| Allele Frequencies, % | ||||||
|---|---|---|---|---|---|---|
| Allele | Nucleotide | Amino Acid Substitution | Enzyme Activity | White | African | Asian |
| TPMT*1 | WT | Wild-type | ||||
| TPMT*2 | 277G→C | 80Alanine→proline | Low | 0-0.7 | 0-0.4 | 0 |
| TPMT*3A | 460G→A | 154Alanine→threonine | Low | 2.24-8.6 | 0-0.8 | 0-0.3 |
| 719A→G | 240Tyrosine→cysteine | |||||
| TPMT*3B | 460G→A | 154Alanine→threonine | Significant decrease on in vitro assay | 0-0.13 | 0 | 0 |
| TPMT*3C | 719A→G | 240Tyrosine→cysteine | Decrease on in vitro assay | 0.1-0.8 | 2.4-10.1 | 0.6-4.75 |
| TPMT*3D | 460G→A | 154Alanine→threonine | Intermediate | > 0.1 | ||
| 719A→G | 240Tyrosine→cysteine | |||||