Key Points
Disease summary:
Esophageal cancers (ECs) are comprised of two main classes, esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC). Although globally ESCC is the more common of the two, in some western countries such as the United States of America and United Kingdom, EAC has become the dominant histology. Particularly high incidence rates of ESCC are observed in Iran, East Asia, and some African regions.
Rarely melanoma, sarcoma, small cell carcinoma, or lymphoma may arise.
More than 80% of the 481,000 yearly cases occur in developing countries; the disease is two to four times more common among males.
EAC is associated with Barrett esophagus (BE) as a consequence of gastroesophageal reflux disease (GERD) and obesity and usually occurs in the distal esophagus. ESCC is associated with smoking and alcohol and typically occurs in the mid and proximal esophagus.
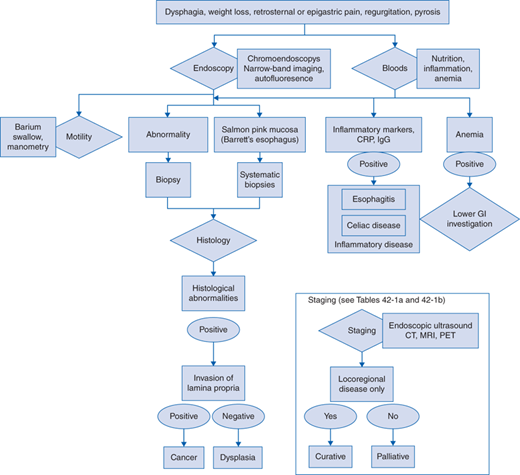
The clinical presentation can usually be attributed to the direct local effects of the disease: dysphagia, sometimes accompanied by pain (odynophagia) and regurgitation of saliva or food. Weight loss is an early feature due to dysphagia and tumor-related cachexia in more advanced stages.
Differential diagnosis:
It includes benign inflammatory esophageal stricture, esophagitis, gastric cancer, and achalasia or other motility disorders.
Monogenic forms:
No single hereditary gene cause of EAC or ESCC is known to exist; however, tylosis is a Mendelian genetic syndrome, inherited in an autosomal dominant manner, and is associated with a high risk of developing ESCC (95% risk of developing ESCC by age of 65 years with type A). Linkage studies have suggested an association with the 17q25 locus tylosis esophageal cancer (TOC).
Family history:
Increased risk of ESCC has been associated with a family history of upper gastrointestinal (GI) cancer probably due to inheritance of multiple low penetrance susceptibility genes. A first-degree relative is associated with one- to threefold increased risk and two or more affected first-degree relatives are associated with a 10-fold increased risk. Although several studies have shown familial aggregation of BE and EACC, linkage analysis in families has not been reported. Familial investigation of EC has primarily been performed in Asian populations; smaller Caucasian studies have shown a lack of evidence for familial susceptibility.
Twin studies:
Although there have been no twin studies on EC, a genetic component to GERD has been demonstrated.
Environmental factors:
The major risk factors for ESCC are tobacco smoking and alcohol consumption. ESCC is also associated with the consumption of burning-hot beverages and pickled foods. The main risk factor for EAC is reflux disease and obesity. There is a weaker risk association of EAC with tobacco smoking.
Genome-wide associations:
Several associations have been identified by genome-wide association studies (GWAS) for ESCC; however, it is not known whether these are functionally important. Testing for single-nucleotide polymorphisms (SNPs) is not yet clinically validated.
Pharmacogenomics:
The understanding and clinical application of genomics to therapy selection lags a long way behind other more common epithelial cancers.
Diagnostic Criteria and Clinical Characteristics
Diagnostic evaluation should include
A history to distinguish true esophageal dysphagia from oropharyngeal problems and likely dysmotility is critical. Clinical examination is mandatory but often noncontributory until in the advanced stages with palpable lymph nodes or liver enlargement. Nutritional status should be assessed.
Blood count and serology, testing for nutritional status, inflammatory markers (eg, C-reactive protein [CRP], immunoglobulin G [IgG]), anemia, liver function.
Endoscopic investigation is the investigation of choice. If there is no obvious lesion, chromoendoscopy utilizing Lugol iodine spray may be of benefit in high incidence areas for ESCC. Narrow band imaging and autofluorescence endoscopy may aid in the detection of early lesions in BE.
Tissue sampling is mandatory to establish the diagnosis. Due to heterogeneity and tumor necrosis six biopsies are recommended. If preinvasive lesions are identified, then multiple biopsy samples should be taken for the evaluation of dysplasia.
Endoscopic ultrasound is useful to evaluate the depth of tumor invasion and lymph node involvement especially in the paraesophageal, gastric, and coeliac regions.
Computed tomography (CT) is essential to provide information on local and systemic spread. Magnetic resonance imaging (MRI) is not currently part of the standard clinical staging algorithm. Positron emission tomography (PET) with fluorodeoxyglucose (FDG) should be used to help identify lymph node metastases and bone spread and may be useful to assess therapy response. CT and PET are increasingly performed as a combined modality.
For tumors around the gastroesophageal junction with a gastric component laparoscopy is usually performed to assess the likely surgical approach (thoracotomy or laparotomy) and check for any peritoneal spread.
| T-stage | |
| TX | Primary cannot be assessed |
| T0 | No evidence of primary |
| Tis | Tumour in situ/high-grade dysplasia |
| T1a | Tumour invades lamina propria or muscularis mucosae |
| T1b | Tumour invades submucosa |
| T2 | Tumour invades muscularis propria |
| T3 | Tumour invades adventitia |
| T4a | Tumour involves adjacent structures: pleura, pericardium, diaphragm, or adjacent peritoneum |
| T4b | Tumour involves other adjacent structures, eg, aorta, vertebral body, trachea |
| N-stage | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | 1 to 2 regional lymph node metastases |
| N2 | 3 to 6 regional lymph node metastases |
| N3 | >6 regional lymph node metastases |
| M-stage | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1a | Metastasis in celiac axis lymph nodes (station 9) |
| M1b | Other distant metastasis |
| Grade | |
| X | Grade cannot be assessed |
| 1 | Well differentiated |
| 2 | Moderately differentiated |
| 3 | Poorly differentiated |
| 4 | Anaplastic |
| Prognostic Group | TNM Staging | Grade | Site | |
|---|---|---|---|---|
| Stage 0 | Tis N0 M0 | 1 | Any | Curative |
| Stage Ia | T1 N0 M0 | 1-2 | Any | Curative |
| Stage Ib | T1 N0 M0, T2 N0 M0 | 3, 1-2 | Lower | Curative |
| Stage IIa | T2 N0 M0 | 3 | Upper/middle | Curative |
| Stage IIb | T1-2 N1 M0, T3 N0 M0 | Any | Lower | Variable |
| Stage IIIa | T4a N0 M0, T3 N1 M0, T1-2 N2 M0 | Any | Upper/middle | Variable |
| Stage IIIb | T3 N2 M0 | Any | Any | Variable |
| Stage IIIc | T4a N1-2 M0, T4b any N M0, any T N3 M0 | Any | Any | Noncurative |
| Stage IV | Any T any N M1 | Any | Any | Noncurative |
Both ESCC and EAC have similar patterns of clinical presentation.
ESCC can present with dysphagia, weight loss, retrosternal or epigastric pain, and regurgitation caused by strictures. Superficial ESCC usually has no specific symptoms, but is sometimes associated with a tingling sensation, it is commonly observed as a slight elevation or shallow depression of the mucosal surface. Histopathologically ESCC is defined as a squamous neoplasm that penetrates the epithelial basement membrane into the lamina propria or deeper tissue layers.
BE is the precursor lesion for EAC but is often clinically silent and may not be apparent in the presence of a large tumor mass. The symptomatology of BE when present is that of GERD usually manifesting as heartburn or acid regurgitation. Following repeated luminal injury the squamous esophageal epithelium is replaced by columnar epithelium. The relative risk of EAC in patients with BE may be less than previously thought but is in the region of 0.33% per annum. In North America and some parts of Europe, the diagnosis of BE is restricted to columnar epithelium with goblet cells, elsewhere gastric metaplasia is also included in the definition.