Key Points
Disease summary:
The classic myeloproliferative neoplasms (MPNs) are a group of clonal hematopoietic stem cell disorders associated with an increased thrombotic tendency and the potential to evolve into acute myeloid leukemia. The central and shared feature in MPNs is effective clonal myeloproliferation with resultant peripheral blood granulocytosis, thrombocytosis, or erythrocytosis that is devoid of dyserythropoiesis, or granulocytic dysplasia. For the sake of this chapter, only the four classic MPNs will be discussed.
Chronic myelogenous leukemia (CML) is characterized by a pathogenic reciprocal chromosomal translocation involving chromosomes 9 and 22, t(9;22).
Polycythemia vera (PV) is a characterized by an absolute increase in red cell mass and often accompanied by leukocytosis, thrombocytosis, and splenomegaly.
Essential thrombocythemia (ET) is characterized primarily by thrombocytosis and an elevated risk for both thrombotic and hemorrhagic consequences.
Primary myelofibrosis (PMF) is characterized by progressive symptomatic splenomegaly, cytopenias, peripheral blood leukoerythroblastosis and dacrocytes, bone marrow fibrosis, and extramedullary hematopoiesis.
Differential diagnosis:
Myelodysplastic syndrome (MDS), acute myeloid leukemia, juvenile and chronic myelomonocytic leukemia, secondary causes of thrombocytosis, erythrocytosis and marrow fibrosis, leukemoid reaction, chronic eosinophilic leukemia, chronic neutrophilic leukemia
Monogenic forms:
CML is believed to be the result of a fusion oncogene involving breakpoint cluster region (chromosome 22) and Abelson leukemia virus (chromosome 9)—t(9;22)(q34;q11). This p210 BCR-ABL oncogene encodes for a constitutively active cytoplasmic tyrosine kinase.
Family history:
A large population-based case-control study conducted in Sweden identified a five- to sevenfold elevated risk of MPNs in first-degree relatives of patients with MPNs supporting the theory of shared susceptibility genes.
Twin studies:
Concordance of disease is not observed between twins.
Environmental factors:
Radiation may play a causative role in some patients as high-dose irradiation after atomic bomb exposure has been linked to an increased risk of developing chronic myeloid leukemia and in vitro exposure to high-dose irradiation can induce BCR-ABL expression in myeloid cell lines. Recently, a statistically significant cluster of cases of PV have been identified in a tri-county area in Eastern Pennsylvania and ongoing investigation of potential toxins present in this area is an area of research.
Genome-wide associations:
There are susceptibility haplotypes that have been identified that increase the risk of developing an MPN. For example, single-marker analysis and haplotype analysis have identified a BCL2 single-nucleotide polymorphism (SNP) that increase the risk for developing CML by an odds ratio (OR) of 1.84 for three to four risk alleles versus zero to one risk alleles. See Table 34-1 for more information.
Pharmacogenomics:
Although not routinely done in clinical practice, OCT-1 activity can be measured in CML CD34+ cells and decreased activity is associated with reduced uptake of imatinib and poor response to therapy. SNPs have been identified in the OCT-1 gene that will likely explain the interperson variability of transporter activity.
| Candidate Gene (Chromosome Location) | Associated Variant [effect on protein] (assayed by affymetrix/illumina) | Risk | Frequency of Risk Allele | Putative Functional Significance | Associated Disease Phenotype |
|---|---|---|---|---|---|
| JAK2 (9p24) | JAK2 exon 12 (rs10974944) | 2.1 OR | 64% | EPOR signaling | PV |
| JAK2 (9p24) | JAK2 V617F (rs12343867) | 1.9 OR | Cytokine receptor signaling | ET, PV, MF | |
| BCL2 (18) | rs1801018 | 2.16 OR | Regulation of apoptosis pathway | CML |
Diagnostic Criteria and Clinical Characteristics
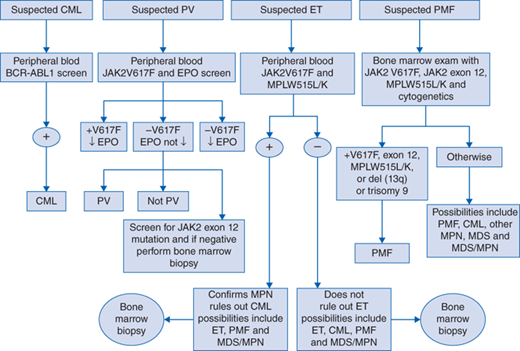
See Fig. 34-1 for both a genetic- and histology-based diagnostic algorithm for classic chronic MPNs. Below are the World Health Organization (WHO) diagnostic criteria for CML and the classic Ph− MPNs.
2008 WHO diagnostic criteria for CML
The presence of Philadelphia chromosome by conventional cytogenetics (karyotyping) or fluorescence in situ hybridization (FISH) analysis, or BCR-ABL1 fusion mRNA by reverse transcription polymerase chain reaction (RT-PCR) in the setting of neutrophilia in all stages of maturation.
2008 WHO diagnostic criteria for PV*
Major Criteria
Hgb greater than 18.5 g/dL (men), greater than 16.5 g/dL (women) or Hgb greater than 17 g/dL(men), greater than 15 g/dL (women) if associated with a sustained increase of at least 2 g/dL from baseline value that cannot be attributed to correction of iron deficiency or Hgb or hematocrit greater than the 99th percentile of reference range for age, sex, or altitude of residence or red cell mass greater than 25% above the mean normal predicted
Presence of JAK2V617F or similar mutation such as JAK2 exon 12
Minor Criteria
Bone marrow trilineage myeloproliferation
Subnormal serum erythropoietin (Epo) level
Endogenous erythroid colony (EEC) growth
*The diagnosis of PV requires meeting either both major criteria and one minor criterion or the first major criterion and two minor criteria.
2008 WHO diagnostic criteria for ET*
Major Criteria
Platelet count greater than or equal to 450 × 109/L
Megakaryocyte proliferation with large and mature morphology, no or little granulocyte or erythroid proliferation
Not meeting WHO criteria for CML, PV, PMF, MDS, or other myeloid neoplasm
Demonstration of JAK2V617F or other clonal marker or no evidence of reactive thrombocytosis
*The diagnosis of ET requires meeting all 4 major criteria.
2008 WHO diagnostic criteria for PMF*
Major Criteria
Megakaryocyte proliferation and atypiaγ accompanied by either reticulin and/or collagen fibrosis, or in the absence of reticulin fibrosis, the megakaryocyte changes must be accompanied by increased bone marrow cellularity, granulocytic proliferation, and often decreased erythropoiesis (ie, prefibrotic PMF).
Not meeting WHO criteria for CML, PV, MDS, or other myeloid neoplasm.
Demonstration of JAK2V617F or other clonal marker or no evidence of reactive bone marrow fibrosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree